An aluminum sphere contains 8.55 * 10 22 aluminum atoms. What is the spheres radius in centimeters?
Question:
An aluminum sphere contains 8.55 * 1022 aluminum atoms. What is the sphere’s radius in centimeters?
The density of aluminum is 2.70 g/cm3.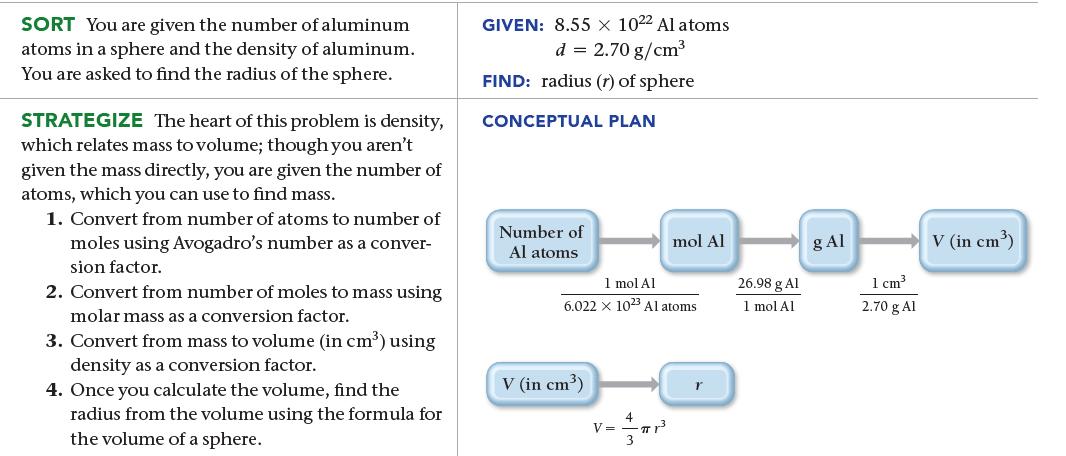

Transcribed Image Text:
SORT You are given the number of aluminum atoms in a sphere and the density of aluminum. You are asked to find the radius of the sphere. STRATEGIZE The heart of this problem is density, which relates mass to volume; though you aren't given the mass directly, you are given the number of atoms, which you can use to find mass. 1. Convert from number of atoms to number of moles using Avogadro's number as a conver- sion factor. 2. Convert from number of moles to mass using molar mass as a conversion factor. 3. Convert from mass to volume (in cm³) using density as a conversion factor. 4. Once you calculate the volume, find the radius from the volume using the formula for the volume of a sphere. GIVEN: 8.55 x 10²2 Al atoms d = 2.70 g/cm³ FIND: radius (r) of sphere CONCEPTUAL PLAN Number of Al atoms 1 mol Al 6.022 x 1023 Al atoms V (in cm³) V = mol Al πTr³ 26.98 g Al 1 mol Al g Al 1 cm³ 2.70 g Al V (in cm³)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
855 x 1022 Al atoms X 4 3 V ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
What is the company' s sustainable growth rate? Suppose the firm targets a 30% sales growth rate next year, and the firm doesn't want to issue new equity, what will be the the firm's new D/A ratio?...
-
The Ebitts Field Corp. manufactures baseball gloves. Charlie Botz, the companys top salesman, has recommended expanding into the baseball bat business. He has put together a project proposal...
-
On July 1,the first day of their fiscal year, the City of Denver sold bonds with a face value of $10,000,000 at 102 percent par. The bonds bear annual interest at 6 percent; interest is payable...
-
Cede & Co. can borrow at 9 percent. Cede currently has no debt, and the cost of equity is 16 percent. The current value of the firm is $540,000. What will the value be if Cede borrows $110,000 and...
-
In this example, you are asked to make appropriate assumptions and model flow past the heat exchanger tubes as shown in Figure 7.54. A schematic diagram of a typical cross flow heat exchanger...
-
Assume the law of the excluded middle is not true, and use this to prove the equality 1 = 0.
-
Lauren, Matthew, and Susan form a partnership, with Lauren contributing $100,000; Matthew $50,000; and Susan her time and skill. Nothing is said regarding the division of profits. The firm later...
-
Suppose we have the following information for an economy: GDP deflator 90 100 110 120 130 Aggregate Expenditure 550 500 450 400 350 Output 150 300 450 600 750 a. Plot the AD and AS curves in a...
-
First Trax Company manufactures snowboards. Its standard cost information follows. First Trax has the following actual results for the month of June: Number of units produced and sold...
-
Write the symbol for each element and classify it as a metal, nonmetal, or metalloid. a. Gold b. Fluorine c. Sodium d. Tin e. Argon
-
A mixture of CaCO 3 and (NH 4 ) 2 CO 3 is 61.9% CO 3 by mass. Find the mass percent of CaCO 3 in the mixture.
-
A sociological study was conducted to determine whether there is a relationship between the length of time blue- collar workers remain in their first job and the amount of their education. From union...
-
74f12) 78: regulon function of Z. P.T DR +34=4
-
How important is a college education for social mobility in the United States? Why? Imagine you were accepted into the best school in the country, but attending meant you would graduate with $120,000...
-
When is 100% of a warrant or right taxed as a capital gain?
-
Short statements reflecting various personality traits which individuals rate on the degree to which they are self-descriptive are referring to as what ? Provide brief explanation.
-
Mr. Scout has an individual share savings account, a share draft account and an IRA, each at a separate branch. The combined balance of these accounts is $500,000. It breaks down this way: ($200,000...
-
One hundred students who had reported that they use their computers for at least 20 hours per week were asked to keep track of the number of crashes their computers incurred during a 12-week period....
-
Represent each of the following combination of units in the correct SI form using an appropriate prefix: (a) m/ms, (b) k m, (c) k s /mg, and (d) k m N.
-
Acid-catalyzed hydration of 1-methylcyclohexene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explane.
-
Calculate S surroundings and S total for part (c) of Problem P5.6. Is the process spontaneous? The state of the surroundings is T = 310.K, P = 0.333 bar.
-
Acid-catalyzed hydration of 1-methylcyclohexene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explane.
-
Create multiple estimates of the intrinsic value of equity for Ford Tesla, Mazda and Volvo, including the use of a dividend discount, model and method of comparable. Compare your estimated values to...
-
Personal Reflection Paper (about thousand words) This reflection paper is meant to assess and reflect on your own personal finance journey. Some of you will be just beginning your journey, others may...
-
A car dealer acquires a used car for $19,000, with terms FOB shipping point. Compute total inventory costs assigned to the used car if additional costs include . $120 for transportation-in. $170 for...

Study smarter with the SolutionInn App


