An LP gas tank in a home barbeque contains 13.2 kg of propane, C 3 H 8
Question:
An LP gas tank in a home barbeque contains 13.2 kg of propane, C3H8. Calculate the heat (in kJ) associated with the complete combustion of all of the propane in the tank.![]()
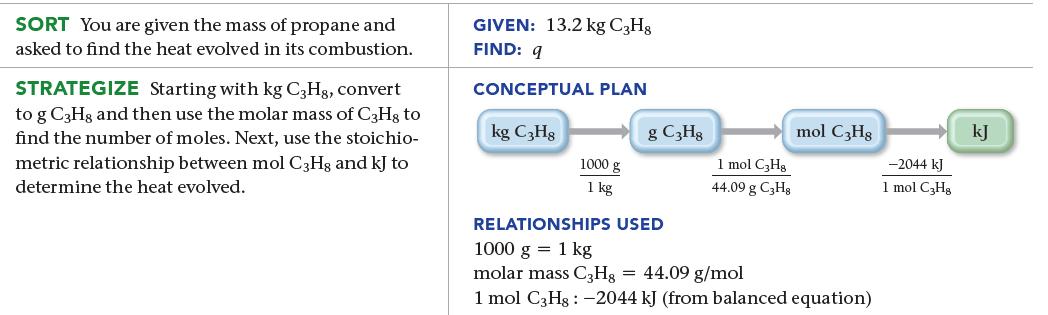
Transcribed Image Text:
C3Hg(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g) ΔΗχη = –2044 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
1000 g 132 kg C3...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A propane tank on a home barbeque contains 10.4 * 10 3 g of propane. a. Write the balanced chemical reaction for the combustion of gaseous propane (C 3 H 8 ) to form water vapor and gaseous carbon...
-
A gas tank in a Toyota Corolla is 13.2 gallons in capacity. How many liters of gasoline when full will this tank hold. Round to nearest hundredth.
-
A rigid tank contains an ideal gas at 40°C that is being stirred by a paddle wheel. The paddle wheel does 200 kJ of work on the ideal gas. It is observed that the temperature of the ideal gas...
-
As we continue our discussions regarding revenue's associated with public sector, this will help reinforce some of the ideas regarding taxes on goods and services. Through the next week, save your...
-
Diewold Company has two departments, Milling and Assembly. The company uses a job-order cost system and computes a predetermined overhead rate in each department. The Milling Department bases its...
-
The following information for Decher Automotives covers the year ended 2010: Administrative expense ............. $ 62,000 Dividend income ............... 10,000 Income taxes .....................
-
The following is the adjusted trial balance of Flappy Health, Inc., for August 31,2010. Requirement 1. Journalize the closing entries at August 31. Happy Health, Inc. Adjusted Trial Balance August...
-
Winter Companys balance sheet at December 31, 2014, is presented below. During January 2015, the following transactions occurred. Winter uses the perpetual inventory method. Jan. 1 Winter accepted a...
-
Access TPB's Code of Professional Conduct for BAS agents as outlined in the Tax Agent Services Act 2009. identify two (2) principles and explain how they relate to payroll operations. Principle How...
-
Consider the following thermochemical equation: What is the heat associated with the reaction of 6 moles of A? (a) -51.0 J (b) -306 J (c) -153 J (d) 153 J 2A AA 51.0J
-
A friend claims to have constructed a machine that creates electricity but requires no energy input. Explain why you should be suspicious of your friends claim.
-
Go to the Enterprise Website (www.enterprise.com) and check out the game, career opportunities, and other components. Then evaluate how effective you feel the Web site is as an employment branding...
-
Provide two examples of strategies you have put in place to support all children to fully participate as valued members of the group (e.g., intentional teaching, use of peer support when...
-
A bakery in a remote town faces essentially no competition. The weekly demand for its famous morning buns is given by 800 - 200p, where p is the price per bun. Currently, the bakery is charging $2.55...
-
1: In what way might a referral/reference mention help in the cover letter process? Please explain. (1 mark) 2: For a job of Accountant Paragraph 3 is an important component of the cover letter....
-
Your existing Natural Gas Furnace has died. A new Natural Gas Furnace with a similar efficiency of 80% to the one that died will cost $5100.00. A new High Efficiency Natural Gas Furnace with...
-
What, in your view, are the fundamental similarities between Kenneth Burke's dramatism and Walter Fisher's narrative paradigm?What distinguishes the two theories? What are the weaknesses of each?
-
Explain the role played by the Export-Import Bank in international trade. Do you consider this bank to be in competition with private lending institutions?
-
Create a data model for one of the processes in the end-of-chapter Exercises for Chapter 4. Explain how you would balance the data model and process model.
-
The prestressed concrete girder is made from plain stone concrete and four -in. cold-form steel reinforcing rods. Determine the dead weight of the girder per foot of its length. 8 in. 6 in. 20 in. 6...
-
The wall is 2.5 m high and consists of 51 mm à 102 mm studs plastered on one side. On the other side is 13 mm fiberboard, and 102 mm clay brick. Determine the average load in kN/m of length of...
-
A building wall consists of exterior stud walls with brick veneer and 13 mm fiberboard on one side. If the wall is 4 m high, determine the load in kN/m that it exerts on the floor.
-
A 1 kg block on a spring is modeled by the following initial value problem. x"+4x= 12 cos(4t), x(0) = 0, x'(0) = 0 a) Find the position of the block at time t x(t) = b) Assuming the forcing function...
-
, 25 An international consortium plans to build the fanciest steakhouse of the world, Westmount Cove, at the foothill of Montreal's Westmount. The restaurant will look like a cave: part of the...
-
Let T and U be independent random variables with P(T = -1) = P(U = P(T = 1) = P(U = 1) = 0.2. Let V = T/U and W = T - U. = -1) == 0.8 and (a) Determine the joint distribution for V and W in the form...

Study smarter with the SolutionInn App


