At 473 K, for the elementary reaction 2 NO(g) + Cl 2 (g) A sample of NOCl
Question:
At 473 K, for the elementary reaction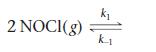
2 NO(g) + Cl2(g)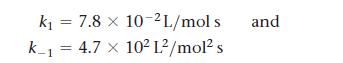
A sample of NOCl is placed in a container and heated to 473 K.
When the system comes to equilibrium, [NOCl] is found to be 0.12 mol/L. What are the concentrations of NO and Cl2?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





