At a given temperature, a system containing O 2 (g) and some oxides of nitrogen can be
Question:
At a given temperature, a system containing O2(g) and some oxides of nitrogen can be described by these reactions: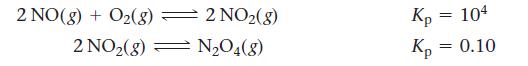
A pressure of 1 atm of N2O4(g) is placed in a container at this temperature. Predict which, if any, component (other than N2O4) will be present at a pressure greater than 0.2 atm at equilibrium.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





