Calculate G rxn for the reaction: Use the following reactions and given G rxn values: CaCO3(s) CaO(s)
Question:
Calculate ΔG°rxn for the reaction:![]()
Use the following reactions and given ΔG°rxn values: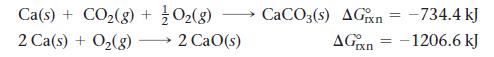
Transcribed Image Text:
CaCO3(s) CaO(s) + COz(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To calculate the standard Gibbs free energy change AG for the reaction CaCO3s CaOs CO2g you can ...View the full answer

Answered By

Junaid ahmed
I am an English language professor with years of experience In Teaching English Language and Literature. I like to help people in the various difficult matter.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
Proper management and leadership are key when dealing with finances in sports. Explain the difference between the two and how they will apply to the financial structure of your sports organization?
-
A builder gets a construction loan of 10 million. For simplicity, assume the loan has an interest rate at j1=6%. The loan does not require payment during the construction process. Construction will...
-
What is the formula to find total dividend and payout ratio? This is the information I have: the amount of shares the company holds and the last dividend paid. Lastly, will there be enough cash to...
-
Discuss how the globalization of markets, especially Europe after 1992, affects retail distribution.
-
Susan Scott has discovered a problem involving the mix of lye to the dry concrete mix that costs the company $20,000 in waste and $14,000 in lost business per period. There are two alternative...
-
After the positrons were annihilated, the energy density of the universe was dominated by the photons and the neutrinos. Show that the energy density in that era was given by \(u_{\text {total...
-
Roley Corporation uses a periodic inventory system and the gross method of accounting for purchase discounts. On July 1, Roley purchased $60,000 of inventory, terms 2/10, n/30, FOB shipping point....
-
1 = We know that all the statements [4] Consider the system (0.3)-(0.4) in the particular case f(r) = proven in Problem [3] apply to this case, so for example, we know that c2 7 = - 1 12 (0.6) de and...
-
Consider the reaction: 2 NO(g) + O2(g) 2 NO 2 (g) Estimate G for this reaction at each temperature and predict whether or not the reaction is spontaneous. (Assume that H and S do not change too much...
-
Determine G for the reaction: Use the following reactions with known G rxn values: FeO3(s) + 3 CO(g) 2 Fe(s) + 3 CO(g)
-
Which is stronger the covalent bond that holds atoms together within a molecule or the dipole- dipole attraction between two neighboring molecules?
-
Discuss the pay structure recommendations given by Nutriment Biotech's human resource manager, Jack Stewart. This discussion will prepare for an upcoming meeting with Nutriment Biotech's owners to...
-
Why do you think Equiano frequently compares his tribe, the Ibo, with the ancient Israelites? How might these comparisons be aimed at his book's primary audience? How do these comparisons help his...
-
How are special subjects used in an alphabetic storage arrangement?
-
What areas to you want to practice with another adult or learn more about before engaging in mentoring about them? Would having a course or a workshop on the topic of observation help you or the...
-
What are some of the stereotypes people hold about people from other cultures? What are some of the stereotypes others hold about you and your cultural group? Where do you believe these stereotypes...
-
1. Company A issued $7,000,000 of a bond on 1-1-x1 for 98 (meaning 98%). The bonds have a 10 year life, stated interest rate of 12%, semiannual interest payments. 2. Company B issued $3,000,000 of a...
-
A glass manufacturer produces hand mirrors. Each mirror is supposed to meet company standards for such things as glass thickness, ability to reflect, size of handle, quality of glass, color of...
-
Consider again the diver in Figure P8.16. Assume the diving board now has a mass of 30 kg. Find the total torque due to gravity on the diving board. Assume the mass of the board is uniformly...
-
Consider the clock in Figure 8.17. Calculate the magnitude and sign of the torque due to gravity on the hour hand of the clock at 4 o???clock. Assume the hand has a mass of 15 kg, a length of 1.5 m,...
-
A rod of length 3.8 m is hinged at one end, and a force of magnitude F = 10 N is applied at the other (Fig. P8.19).? (a) If the magnitude of the torque associated with this force is 18 N m, what is...
-
You want to be sure to consider how consumers would make a decision to join The Last 5 Pounds (described above). Demonstrate your understanding of the consumer decision-making process by elaborating...
-
Carla Vista Ltd., a private company reporting under ASPE, reported the following for the years ended May 31, 2024 and 2023. CARLA VISTA LTD. Balance Sheet May 31 Assets 2024 2023 Cash $12,100 $41,280...
-
A mining company is considering investing in a low-grade placer deposit of gold ore. The project will require $22 million initial investment and is expected to generate profits of $5 million per year...

Study smarter with the SolutionInn App


