Calculate how many moles of NO 2 form when each quantity of reactant completely reacts. 2 NO5(g)
Question:
Calculate how many moles of NO2 form when each quantity of reactant completely reacts.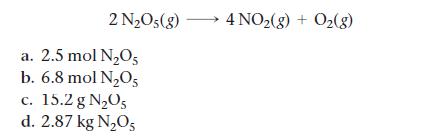
Transcribed Image Text:
2 N₂O5(g) a. 2.5 mol N₂O5 b. 6.8 mol N₂O5 c. 15.2 g N₂O5 d. 2.87 kg N₂O5 4 NO₂(g) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a 50 mol N...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate how many moles of NH 3 form when each quantity of reactant completely reacts. 3 NH4(1) a. 2.6 mol NH4 c. 65.3 g NH4 4 NH3(g) + N(8) b. 3.55 mol NH4 d. 4.88 kg NH4
-
Aluminum hydroxide reacts with sulfuric acid as follows: Which is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2SO4 are allowed to react? How many moles of Al2(SO4)3 can form under...
-
Procedures Experiment 1: Standardize the Iodine Solution Part 1: Prepare the Materials Take a 100 mL volumetric flask from the Containers shelf and place it on the workbench. Take ascorbic acid from...
-
Accounting The Case: Patient Khaled is a 75-year-old man admitted to the hospital for a small bowel obstruction. His medical history includes hypertension. Khaled is on NPO. He has a nasogastric (NG)...
-
What is the primary disadvantage of the of the corporate form of organization? Name at least two of the advantages of corporate organization.
-
Introducing Yourself to Your Instructor
-
Think of a time when you have been very satisfied with a job you have held. What made that job satisfying? Also think of a time when you have been dissatisfied with a job you have held. What made...
-
Finn Fixes is a new charity that repairs donated cars for use by unemployed job seekers. Finn gets the cars for free from churches in the area. The churches collect the used cars from their...
-
Before you begin this exploration of the most utilized therapies that counselors employ in their work, please share what has worked for you in your own life when you have had to make changes. How did...
-
An entertainment company has a net income goal of $19,000/Month. Sales are currently $600,000 annually and fixed costs run $95,000 each year? The company has just launched a new brand campaign that...
-
Consider the balanced equation: Complete the table showing the appropriate number of moles of reactants and products. If the number of moles of a reactant is provided, fill in the required amount of...
-
Consider the unbalanced equation for the neutralization of acetic acid: Balance the equation and determine how many moles of Ba(OH) 2 are required to completely neutralize 0.461 mole of HC 2 H 3 O 2...
-
Suppose the balance sheets of a corporation for two years reported these figures: The notes to the 2014 financial statements report that during 2014, because of some refinancing arrangements, the...
-
Anthropomorphic elements generally are not acceptable to include in predictions regarding animal behavior. Which two predictions below include anthropomorphic elements?
-
Investigate the Adding Fractions eManipulative. A common denominator is needed to add two fractions like the ones below. Find the sum of the fractions represented in the pictures. (Simplify your...
-
14. If m" n" 5184, then the value of m+nis = 15. If 52 +5+5 651, then 9"=
-
Question: think that you are being hired by a World Bank task force to identify opportunities to reframe one of the issues mentioned above and design a solution to address one of the UN SDGs of good...
-
(a) Let (3.9.6) and = (1.1.2). (i) Find Proj. the orthogonal projection of onto . (ii) Using your answer from part (a), write if=+where r for some rER. and 0. [4] (b) Use the method of least squares...
-
1. Given the information in the case, which Incoterms group (E, F, C, or D) should ME pursue as the exporter? Why? 2. Based on your response to Question 1, what responsibilities and risks will ME...
-
Chicago Company sold merchandise to a customer for $1,500 cash in a state with a 6% sales tax rate. The total amount of cash collected from the customer was $558. $600. $642. $636. Nevada Company...
-
A hard-working horse can lift a 350. lb. weight 100. ft. in one minute. Assuming the horse generates energy to accomplish this work by metabolizing glucose: C 6 H 12 O 6 (s) + 6O 2 (g) 6CO 2 (g) +...
-
Under what conditions is the distribution of products in an ideal gas reactions system at equilibrium unaffected by an increase in the pressure?
-
Identify which of the following compounds is most activated toward electrophilic aromatic substitution. Which compound is least activated? Br NO2 NO2 OMe .
-
How to find NPV in lease and buy case analysis. Daiwan Semiconductor Manufacturing Company Limited ( the Client ) , together with its subsidiaries, manufactures, packages, tests, and sells integrated...
-
Consider an annual coupon bond with a face value of $100,8 years to maturity, and a price of $77..The coupon rate on the bond is 7%.If you can reinvest coupons at a rate of 1% per annum, then how...
-
Kroger Inc. is considering a project that has the following cash flow data. What is the project's payback? Year 0 1 2 3 Cash flows -$450 $200 $200 $200 2.39 years 1.94 years 1.71 years 2.25 years...

Study smarter with the SolutionInn App


