Carbon monoxide and chlorine gas react to form phosgene (COCl 2 ) according to the equation: The
Question:
Carbon monoxide and chlorine gas react to form phosgene (COCl2) according to the equation:![]()
The rate law for the reaction is Rate = k[Cl2]3/2[CO]. Which representation of a mixture of chlorine gas and carbon monoxide gas has the fastest initial rate?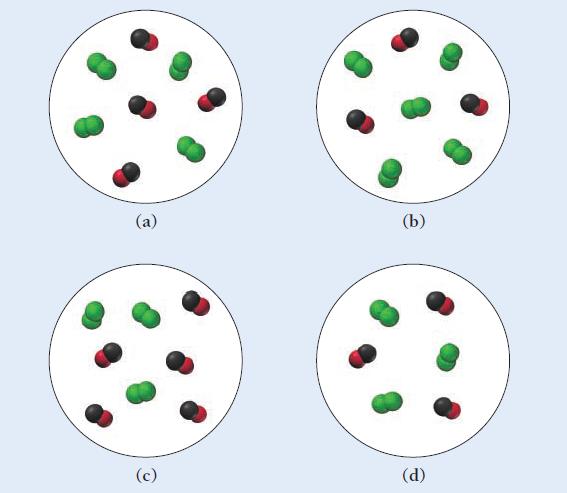
Transcribed Image Text:
CO(g) + Cl₂(g) - COCI₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Nitu Kapoor
i Hv done my education from dev samaj college for women fzr n hv taught in two different colleges with one year experience in each. One year in mata sahib kaur college n one year in DAV college for women fzr.I was also a tutor at home.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Sonam newly purchased Brand Elo skincare lotion. She compared her perception of how the Lotion made her skin glow and fresh to her expectations regarding Brand Elo skincare lotion. Sonam was...
-
Carbon monoxide and chlorine gas react to form phosgene: If a reaction mixture initially contains 215 torr of CO and 245 torr of Cl 2 , what is the mole fraction of COCl 2 when equilibrium is...
-
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: (a) What is the value of Kp at this temperature? (b) Does the equilibrium favor reactants or products? (c)...
-
True Or False Death benefits are used to compensate the deceaseds family for pain and suffering.
-
Factory labor data for Weber Company are given in BE3-2. Manufacturing overhead is assigned to departments on the basis of 200% of labor costs. Journalize the assignment of overhead to the Assembly...
-
A 10-kg mass of superheated refrigerant-134a at 1.2 MPa and 70C is cooled at constant pressure until it exists as a compressed liquid at 20C. (b) Determine the change in volume. (c) Find the change...
-
Which of the following is not appropriate for the accountant's report on the results of applying agreed-upon procedures to prospective financial statements? a. State the accountant's opinion on the...
-
The purchasing manager for the Pacific Steel Company must determine a policy for ordering coal to operate 12 converters. Each converter requires exactly 5 tons of coal per day to operate, and the...
-
A firm has fixed assets with a book value of $12,000 and a useful life of three-years. If pretax accounting income is $25,000 this year, $20,000 next year and $35,000 in the following year provide...
-
Explain how a chemical reaction occurs according to the collision model. Explain the meaning of the orientation factor in this model.
-
What is an Arrhenius plot? Explain the significance of the slope and intercept of an Arrhenius plot.
-
In addition to the process described in the text, a second process called the carbon-nitrogen cycle occurs in the sun: a. What is the catalyst in the above scheme? b. What nucleons are intermediates?...
-
Hemming Company reported the following current - year purchases and sales for its only product. Date Activities Units Acquired at Cost Units Sold at Retail January 1 Beginning inventory 2 8 0 units @...
-
The records for Kalman Ltd . show the following data for calendar 2 0 2 3 : 1 . Gross profit on instalment sales recorded on the books was $ 1 0 0 , 0 0 0 . Gross profit from collections of...
-
The following information is available for Pyle Garage for March, Year 2 : BANK STATEMENT HAZARD STATE BANK 2 1 5 MAIN STREET HAZARD, GA 3 0 3 2 1 Pyle Garage 6 2 9 Main Street HAZARD, GA 3 0 3 2 1...
-
During the month of June, Ace Incorporated purchased goods from two suppliers. The sequence of events was as follows: June 3 Purchased goods for $ 4 , 2 0 0 from Diamond Incorporated with terms 3 / 1...
-
Shown here are annual financial data for a merchandising company and a manufacturing company. Music World Retail Wave - Board Manufacturing Beginning inventory Merchandise $ 1 3 5 , 0 0 0 Finished...
-
What is activity-based management? How is it different from activity-based costing?
-
The pendulum consists of two rods: AB is pin supported at A and swings only in the y-z plane, whereas a bearing at B allows the attached rod BD to spin about rod AB. At a given instant, the rods have...
-
A compact disc spins at 2.5 revolutions per second. An ant is walking on the CD and finds that it just begins to slide off the CD when it reaches a point 3.0 cm from the CDs center. (a) What is the...
-
A heavy crate is pushed across a level floor at a constant velocity. What is the total work done on the crate by all forces? How does your answer change if the crate is being pushed up a hill?
-
A car of mass m = 1500 kg is pushed off a cliff of height h = 24 m (Fig. P6.28). If the car lands a distance of 10 m from the base of the cliff, what was the kinetic energy of the car the instant...
-
Given the points P(-1,3,-7), Q(0,4,-5), and R(-3,6,-1). (a) Find v, a vector of length 5 in the direction of PQ. (b) Find sin, where is the angle between the vectors PQ and PR. Do not actually...
-
*Clearly stated the name of the company and provided a thorough explanation describing what they do. *Included and explained at least 3 of the following aspects: the core business the company is in,...
-
Carrico Advertising Inc. performs advertising services for several Fortune 500 companies. The following information describes Carrico's activities during the current year. a. At the beginning of the...

Study smarter with the SolutionInn App


