Consider a 0.10 M solution of a weak polyprotic acid (H 2 A) with the possible values
Question:
Consider a 0.10 M solution of a weak polyprotic acid (H2A) with the possible values of Ka1 and Ka2 given here.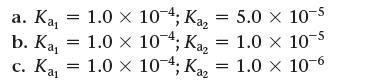
Calculate the contributions to [H3O+] from each ionization step. At what point can the contribution of the second step be neglected?
Transcribed Image Text:
a. Kay b. Ka = c. Kai = = 1.0 × 104; Ka₂ = 5.0 × 10-5 1.0 x 10;Ka₂ = 1.0 × 10-5 1.0 × 10-4; Kaz = 1.0 x 10-6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the contributions to H3O from each ionization step and the point at which the contribut...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The owner of a retail store randomly selected the following weekly data on profits and advertising cost. Week Advertising Cost ($) Profit ($) 1 0 205 2 50 265 3 250 425 4 150 295 5 125 330 Write down...
-
As part of its Economic Response Plan, the Canadian government introduced Canada Emergency Response Benefit (CERB) where a taxable benefit of $2000 a month is provided for up to 4 months to eligible...
-
What is TQM? At what point can a firm consider its effort to achieve total quality management complete?
-
Consider a spherical fluid particle in an inviscid fluid (no shear stresses). If pressure and gravitational forces are the only forces acting on the particle, can they cause the particle to rotate?...
-
Refer to the data in Problem 6-23. Required: Prepare a production report for each department using the FIFO method.
-
Consider the PD control system shown in Figure 10.7.1. Suppose that I = 20 and C = 10. The specifications require the steady-state error due to a unit-step command to be zero and the steady-state...
-
Recording and processing information about a transaction at the time it takes place is referred to as which of the following? a. batch processing b. online, real-time processing c. captured...
-
Assume that Suzie Whitson has decided to begin production of her outdoor childrens toy. Her company is Jiffy Jet and costs for last month follow. Factory rent ................ $ 3,200 Company...
-
What is all math expression for calculation of variable factory overhead controllable variance
-
Calculate the [H 3 O + ] and pH of each H 2 SO 4 solution. At approximately what concentration does the x is small approximation break down? a. 0.50 M b. 0.10 M c. 0.050 M
-
Calculate the concentration of all species in a 0.155 M solution of H 2 CO 3 .
-
Let \((\mathcal{L},\langle\cdot, \cdotangle)\) be a Hilbert space and \(\mathcal{H}\) some linear subspace. Show that \(\mathcal{H}\) is a dense subset of \(\mathcal{L}\) if, and only if, the...
-
Donny Accounting provides accounting services. During the month of May 2022, the following transactions took place. May 1 Borrowed $6,000 cash from the bank. May 3 Provided services to a customer and...
-
What are the molecular mechanisms underlying the adaptive immune response, including somatic recombination, clonal selection, and immunological memory, and how do these processes enable vertebrates...
-
Relative Valuation Method Financial Information Earthlink (ELINK) Yahoo! (YHOO) eBay (EBAY) Microsoft (MSFT) 2003 shares outstanding 159,399,000 655,602,000 646,819,000 10,800,000,000 2003 fiscal...
-
Question 1A (3 marks) EXCEL, an equipment costing $100,000 has a useful life of 6 years. It has a resale value of $8,000. Annual costs will be $7,000 for each of the year. Another equipment, APLUS...
-
T merges into P under state law. T shareholders receive $ 4 0 0 , 0 0 0 of P stock and $ 1 0 0 , 0 0 0 of cash. T shareholder basis in their stock is $ 3 2 5 , 0 0 0 . How much gain do T shareholders...
-
On a typical night in New York, about 25,000 people attend a Broadway show, paying an average price of more than $75 per ticket. Variety (www.variety.com), a news weekly that reports on the...
-
If a and b are positive numbers, find the maximum value of f ( x ) = x a (9 x ) b on the interval 0 x 9.
-
Prove that the determinant of the system matrix in Eq. (6.31) is equal to 1. Dzdy -D1 D2 + a12 Did1 di 1 A22 a21 j (6.31)
-
Establish that Eqs. (6.41) and (6.42) are equivalent to Eqs. (6.3) and (6.4), respectively. ii (1 ) (6.41a) VH| -d12 aj1) VH1 (6.41b) -dj2
-
Show that the planar surface of a concave-planar or convex-planar lens doesnt contribute to the system matrix.
-
TWO-(I4 marks) Generation Meats is a butcher shop owned and operated by Clara Pietr On 1 May 2015 she presented the following information : Cash balance brought forward $ 475 Bank balance brought...
-
Lester's Gym wants to expand its facilities by obtaining $62,000 worth of new exercise equipment. The equipment will have a 4-year life, belongs in a 25% CCA class, and will have no residual value....
-
Garcia Industries uses a cost system that carries direct materials inventory at a standard cost. The controller has established these standards for the cost of one unit: Standard Quantity X Standard...

Study smarter with the SolutionInn App


