Consider the halogenation of ethene, where X is a generic halogen: Use bond energies to determine which
Question:
Consider the halogenation of ethene, where X is a generic halogen:![]()
Use bond energies to determine which halogen produces the most exothermic halogenation reaction with ethene.
The C—F, C—Br, and C—I bond energies are 552 kJ/mol, 280 kJ/mol, and 209 kJ/mol, respectively. Look up all other necessary bond energies in Table 10.3.
a) Fluorine
b) Chlorine
c) Bromine
d) Iodine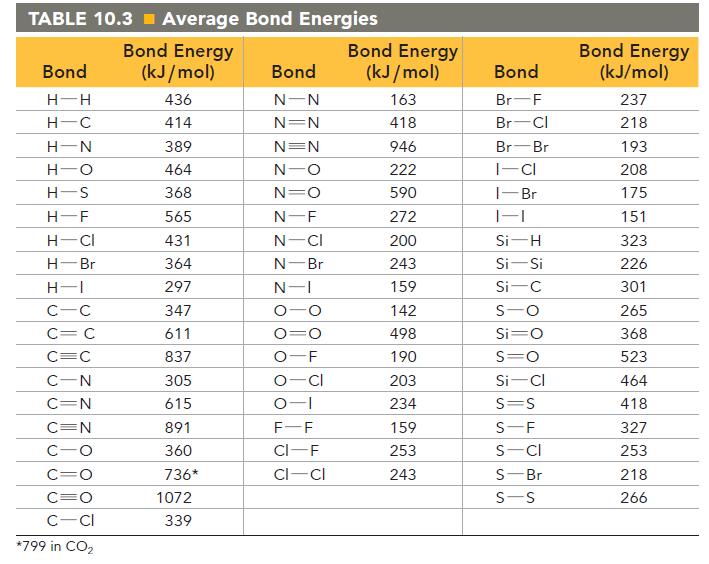
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





