Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following
Question:
Consider the reaction: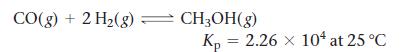
Calculate ΔGrxn for the reaction at 25 °C under each of the following conditions:
a. Standard conditions
b. At equilibrium
c. PCH3OH = 1.0 atm; PCO = PH2 = 0.010 atm
Transcribed Image Text:
CO(g) + 2 H₂(g) = CH3OH(g) Kp = 2.26 x 104 at 25 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a 24...View the full answer

Answered By

Chiranjib Thakur
I have no tutoring experience yet, but I can share my skills and knowledge gained from my education and work experiences. I have been a CPA since 2012 with 6 years of work experience in internal auditing and 4 years of work experience in accounting at the supervisory level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Ray Holt Corporation has retained you as a consultant on accounting policies and procedures. During 2019, the company engaged in a number of treasury stock transactions, having foreseen an...
-
A voltaic cell employs the following redox reaction: Calculate the cell potential at 25 C under each set of conditions. Sn+ (aq) + Mn(s) 2+ Sn(s) + Mn+ (aq)
-
A voltaic cell employs the redox reaction: Calculate the cell potential at 25 C under each set of conditions. 2 Fe+ (aq) + 3 Mg(s) - 2 Fe(s) + 3 Mg+ (aq)
-
Is an oil pipeline a simplex system, a half-duplex system, a full-duplex system, or none of the above? What about a river or a walkie-talkie-style communication?
-
Which of the following products would typically be accounted for using a job order costing system? Which would typically be accounted for using a process costing system? (a) Standard nails, (b)...
-
Refer to the information provided in P2-4. Required Identify each transaction in P2-4 as financing, investing, or operating.
-
Redesign the VOCs adsorber of Example 9.15 for a breakthrough time of \(4.0 \mathrm{~h}\). The pressure drop through the bed [calculated using the Ergun equation (2-95)] should not exceed \(1.0...
-
Rachel Rey recently opened her own basketweaving studio. She sells finished baskets in addition to the raw materials needed by customers to weave baskets of their own. Rachel has put together a...
-
Capitalized Interest (LIVELY ACE Ch 9 Pt 2) On January 1, Year 1, Romano Tire Service, Inc. signed a contract to have a new service center built for $900,000. On the same day, Romano borrowed...
-
Consider the reaction: The following data show the equilibrium constant for this reaction measured at several different temperatures. Use the data to find H rxn and S rxn for the reaction. H(g) +...
-
Estimate the value of the equilibrium constant at 655 K for each reaction in Problem 74. (H f for BrCl is 14.6 kJ/mol.) Problem 74 Use data from Appendix IIB to calculate the equilibrium constants at...
-
Are there certain kinds of advertising campaigns and goals where search advertising wouldn't be a good fit? Give examples and explain why.
-
A full time school teacher recognized a $26000 loss in 2020 from his part time farming activities. What is the maximum deduction allowed in 2020 for the farm loss? Johnny Jobul is self employed and...
-
After listening to "War of the Worlds" in this module's Power Point, think about the repercussions of this news story as it relates to the consequences of "fake news" that we see today. In modern...
-
Lease term is 9 years ABC Lessee Co. pays initial direct costs of $2,350. The lease term is non-cancelable, requiring equal payments due at the beginning of each year in the amount of $4,175. XYZ Co....
-
"In a Canadian population, 30% of people spend 4 hours or more per day on smartphone; 25% of people have difficulty falling asleep within 30 min; and 20% of people spend 4 hours or more per day on...
-
What are the incremental breakeven unit sales for the 10% price cut? If ABC's monthly order is estimated to increase by no more than 24% after the 10% price cut, should ABC implement the price cut?
-
Education is an example of a positive externality: acquiring more education benefits the individual student and having a more highly educated work force is good for the economy as a whole. The...
-
Complete problem P10-21 using ASPE. Data from P10-21 Original cost ................................................................. $7,000,000 Accumulated depreciation...
-
An aluminum sphere has a radius of 45 cm on the Earth. It is then taken to a distant planet where the atmospheric pressure P is much larger than on the Earth. If the sphere has a radius of 43 cm on...
-
Two bars, one composed of aluminum and one of steel, are placed end to end as shown in Figure P11.56. The bars are both initially of length L = 25 cm, and both have a square cross section with h =...
-
The steel cylinder in Figure P11.53A is replaced by a steel tube (Fig. P11.53B). The outer radius of the tube is R 1 = 3.5 cm, and the inner radius is R 2 = 1.5 cm. If the compressive force is F =...
-
Using the following year-end information for Blackstone, LLC, calculate the quick ratio: Cash $ 66,400 Short-term investments 12,400 Accounts receivable 48,500 Inventory 240,000 Prepaid expenses...
-
If the owner withdrew no assets during the year but invested an additional $96,000 in cash, we need to adjust the calculation for the net profit earned. This additional investment would increase the...
-
The following financial data were adapted from recent financial statements for Water Source, Inc.: Sales $27,800 Operating assets: Property, plant, and equipment 17,800 Intangibles 1,200. Calculate...

Study smarter with the SolutionInn App


