Consider the reaction: Each of the entries in the following table represents equilibrium partial pressures of A
Question:
Consider the reaction:
![]()
Each of the entries in the following table represents equilibrium partial pressures of A and B under different initial conditions.
What are the values of a and b in the reaction?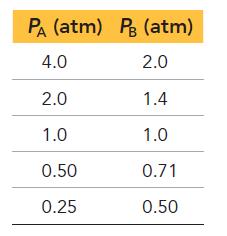
Transcribed Image Text:
aA(g) bB(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Fig P2.40 the pressures at A and B are the same, 100 kPa. If water is introduced at A to increase pA to 130 kPa, find and sketch the new positions of the mercury menisci. The connecting tube is a...
-
You have been assigned the task of measuring the equilibrium constant for the reaction N 2 O 4 2NO 2 as a function of temperature. To do so, you evacuate a rigid 2-liter vessel equipped with a...
-
The equilibrium constant (KP) for the formation of the air pollutant nitric oxide (NO) in an automobile engine at 530°C is 2.9 Ã 10-11: (a) Calculate the partial pressure of NO under these...
-
Refer to the RMO CSMS Order Fulfillment subsystem shown in Figure. Draw a use case diagram that shows all actors and all use cases. Use a drawing tool such as Microsoft Visio if it is available.
-
Pittsburgh-Walsh Company (PWC) is a manufacturing company whose product line consists of lighting fixtures and electronic timing devices. The Lighting Fixtures Division assembles units for the...
-
In the steady state, the charge on the 5-?F capacitor in the circuit in Figure is 1000 ?C. (a) Find the battery current. (b) Find the resistances R1, R2, and R3. 5 R3 5 A R2 10 50 5 R1 310 V
-
Why is an ethical culture considered necessary for fraud prevention and deterrence?
-
Your client took a complete physical inventory count under your observation as of December 15 and adjusted the inventory control account (perpetual inventory method) to agree with the physical...
-
(5 pts) In early 2020, The Week reported that the median wage for Costco's employees is almost $39,000. This is higher than the median wage for all Americans and much higher than Walmart's median...
-
A particular reaction has an equilibrium constant of K p = 0.50. A reaction mixture is prepared in which all the reactants and products are in their standard states. In which direction does the...
-
The reaction A(g) 2 B(g) has an equilibrium constant of K c = 1.0 at a given temperature. If a reaction vessel contains equal initial amounts (in moles) of A and B, does the direction in which the...
-
The probability that a Canadian adult has taken some instruction in canoeing is 0.03. The probability that a Canadian adult who has taken some instruction in canoeing will go on a canoe trip this...
-
The component of the project management plan that describes how project communications will be planned, structured, and monitored is the: a. communication model b. communications management plan c....
-
When this project was envisioned, it was possible to state a vision for the outcomes, but way too premature to try to describe specific outputs. Therefore, it made sense to use an agile approach....
-
Why do so many oligopolies exist in healthcare?
-
It is December of 2021. The NHL Health System has many decisions to make. Its capital budgeting process requires that the largest capital investments, those more than $5 million, be approved by the...
-
You are given a project to manage. How do you decide whether to use a plan-driven or adaptive approach?
-
Victory Company uses weighted- average process costing to account for its production costs. Direct labor is added evenly throughout the process. Direct materials are added at the beginning of the...
-
What is the ideal number of children to have? This question was asked on the Sullivan Statistics Survey I. Draw a dot plot of the variable Children from theSullivanStatsSurveyI data set at...
-
Figure 9.27 shows a duct in which methyl alcohol at 25°C flows at the rate of 3000 L/min. Compute the energy loss over a 2.25-m length of the duct. All surfaces are smooth plastic. 100 mm 30 mm...
-
In Fig. 9.26, ethylene glycol (sg = 1.10) at 77°F flows around the tubes and inside the rectangular passage. Calculate the volume flow rate of ethylene glycol in gal/min required for the flow to...
-
Compute the energy loss for the flow of water in the cooling passage described in Problem 9.34 if its total length is 45 in. Use for steel. Also compute the pressure difference across the total...
-
Please choose a communication technology and examine how it grabs people's attention. How might that impact people and society?
-
1) Calculate Balance in T-Account Dee Bell Company's records showed the following April 30, 2023, account balances: Cash Apr 30 15,000 Accounts Receivable Car Apr 30 3,200 Accounts Payable Unearned...
-
Why is it important that most is that state universities need to be operationally much more self-sustaining than in the past in addition to schools controlling their own financial future independent...

Study smarter with the SolutionInn App


