Consider the reaction for the decomposition of hydrogen disulfide: A 0.500-L reaction vessel initially contains 1.25 *
Question:
Consider the reaction for the decomposition of hydrogen disulfide: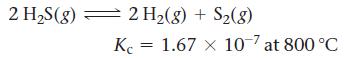
A 0.500-L reaction vessel initially contains 1.25 * 10-4 mol of H2S at 800 °C.
Find the equilibrium concentrations of H2 and S2.
Transcribed Image Text:
2 H₂S(g) 2 H₂(g) + S₂(8) Kc = 1.67 x 107 at 800 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
HS 125 x 104 mol 0500 L 250 x 104 M 2 HSg Initial Change Equil 2 Hg S8 HS 250 x 104 H S 000 000 By i...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the reaction for the decomposition of hydrogen disulfide: A 0.500-L reaction vessel initially contains 0.0125 mol of H 2 S at 800 C. Find the equilibrium concentrations of H 2 and S 2 . 2...
-
Consider the reaction for the formation of H2O(g) from its elements. Use the data in Appendix C to answer the following questions. a. What is the standard enthalpy of formation of H2O(g) at 25oC? b....
-
The following equilibrium was studied by analyzing the equilibrium mixture for the amount of H2S produced. A vessel whose volume was 2.50 L was filled with 0.0100 mol of antimony(III) sulfide, Sb2S3,...
-
Consider the following information for a company: Sales (all credit): $6,000,000; Cost of Goods Sold: 80% of Sales; Accounts receivable: $350,000; Inventory: $600,000; Accounts payable: $150,000 What...
-
Azari, Inc. has just created five order fulfillment value streams, two focused and three that produce multiple products. The size of the plant in which the values streams are located is 150,000...
-
A 1 m3 rigid tank has propane at 100 kPa, 300 K and connected by a valve to another tank of 0.5 m3 with propane at 250 kPa, 400 K. The valve is opened and the two tanks come to a uniform state at 325...
-
From a historical perspective, identify three precedented systems that were replaced with unprecedented systems.
-
James Streets son, Harold, is 10 years old today. Harold, a studious young fellow, is already making plans to go to college on his 18th birthday, and his father wants to start putting money away now...
-
Cullumber Company is considering a long-term investment project called ZIP. ZIP will require an investment of $104,000. It will have a useful life of 4 years and no salvage value. Annual revenues...
-
What is the value of Q when each reactant and product is in its standard state?
-
What is the definition of the reaction quotient (Q) for a reaction? What does Q measure?
-
Water at an average temperature of 300 K flows at 0.7 kg/s in a 2.5-cm-diameter tube 6 m long. The pressure drop is measured as 2 kPa. A constant heat flux is imposed, and the average wall...
-
Suppose Peter retires at age 60 with $1,000,000 in pension. How much will he and his beneficiaries receive if he requests 3 equal periodic payments with the first one to be received at age 60 and the...
-
LG just paid a $2 annual dividend on its common stock. The dividend is expected to increase 5.5% per year indefinitely. If the required rate of return is 7.8%, What is the the stock's value next year?
-
A company purchased a van at the beginning of the year with a cost of $50,500. The useful life is estimated to be 10 years with a $5,000 salvage value and the company uses the straight-line method of...
-
You, as an investor, are offered an investment idea that earns $605.60 in six years if you deposit some amount of money today at an annual 6.50% interest rate compounded semi-annually. How much do...
-
4. [20pts] Debug the following code by hand. Identify all errors, and whether they are a syntax or logic error (comments indicate how the code should work). int x=2, Y=4; Byte Z = -350; for (I=1; I...
-
Assume you are analyzing the financial statements of ABEX Chemicals. Your analysis raises concerns with certain accounting procedures that potentially distort its operating results. Required: a. Data...
-
A liquid flows upward through a valve situated in a vertical pipe. Calculate the differential pressure (kPa) between points A and B. The mean velocity of the flow is 4.1 m/s. The specific gravity of...
-
Calculate v 1 and v 2 in the circuit of Fig. 3.62 using nodal analysis. 600 V V2 10 i +) 50 (4) 15 A 30
-
Using nodal analysis, find v o in the circuit of Fig. 3.63 . 12.5 A 50 V Vo 100 V (+
-
Apply nodal analysis to find i o and the power dissipated in each resistor in the circuit of Fig. 3.64 . 12.5 A 50 V Vo 100 V (+
-
If x has the dimension of [Z], t has the dimension of [7], and v has the dimension of the dimension and the SI unit of the constants A and B in the expression below. v = At + Bt [2] determine
-
During lunch, Timothy and his friends play a game of marbles. In the bag there are 4 blue marbles and 5 purple marbles. What is the probability that a blue marble is randomly chosen, followed by a...
-
40. Riot signed up with an online music-sharing network. The network charges customers $25.00 per month but pays customers $0.02 for every song that they upload from their music collection. Riot's...

Study smarter with the SolutionInn App


