Consider the titration curves (labeled a and b) for two weak bases, both titrated with 0.100 M
Question:
Consider the titration curves (labeled a and b) for two weak bases, both titrated with 0.100 M HCl.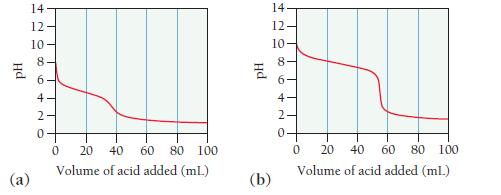
i. Which base solution is more concentrated?
ii. Which base has the larger Kb?
Transcribed Image Text:
Hd (a) 14 == 208 12 10 6 4 N 0 0 20 40 60 80 100 Volume of acid added (mL) PH (b) 14. 12- 10- 00 10 8 6- 4- 2. 0 0 20 40 60 80 100 Volume of acid added (ml.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The base solution in curve a is more concentrated than the base solution in curv...View the full answer

Answered By

David Ngaruiya
i am a smart worker who concentrates on the content according to my clients' specifications and requirements.
4.50+
7+ Reviews
19+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A mountain-climbing expedition establishes two intermediate camps, labeled A and B in the drawing, above the base camp. What is the magnitude (r of the displacement between camp A and camp B? 4900 m...
-
A chemical company produces amongst its product range two industrial cleaning fluids, A and B. These products are manufactured jointly. Total sales are expected to be restricted because home trade...
-
The figure in the preceding exercise shows the pH curves for the titrations of six different acids with NaOH. Make a similar plot for the titration of three different bases with 0.10 M HCl. Assume...
-
How does ESMA support national regulators in enforcing the application of IFRS?
-
What is cycle time? Velocity?
-
1. What are the causes of the confusion confronting Keith Houck? Is H.I.D. ready to formulate a strategic plan? Why or why not? 2. If you were Keith Houck, what questions would you ask the managers?...
-
Consider PowerBar, the strategy for which is summarized in the PowerBar case. What implications for the culture, structure, systems, and people would you suggest given the nature of the product and...
-
Lazy River Resort opened for business on June 1 with eight air-conditioned units. Its trial balance before adjustment on August 31 is as follows. In addition to those accounts listed on the trial...
-
K Use appropriate formulas to find (a) the perimeter and (b) the area of the figure. 8.8 m 5 m 5.2 m 7 m (a) The perimeter is (Type an integer or a decimal.)
-
Phenolphthalein has a pK a of 9.7. It is colorless in its acid form and pink in its basic form. For each of the values of pH, calculate [In ]/[HIn] and predict the color of a phenolphthalein...
-
Calculate G and K for each reaction the group created in Question 143. For one of the reactions, explain how the sign or magnitude of each quantity (Ecell, G, and K) is consistent with the fact that...
-
How might an employer create an implied-in-fact term and how could a failure to follow such policies when terminating an employee create a breach of the contract? Issue : Whether there is an implied...
-
Examine two drawbacks and two benefits associated with ISO 9000 certification, delineating its implications within organizational frameworks.
-
Cam is really mindful that keeping on top their cash is really important and has been taking note of their cash balances but is confused as to why their online bank balance never seems to match with...
-
23 2. 2 === Complete the following equations: + + + i 2 22 26 = + i 2001 = + Hint: All you need to know to solve this problem is that i = -1.
-
You bring your friend to the Biomechanics Lab and tell them to stand naturally in a static position on the force plate. The force plate measures their mass at 78 kg and measures their right foot 21...
-
Project A costs $1,000, and its cash flows are the same in Years 1 through 10. Its IRR is 16%, and its WACC is 11%. What is the project's MIRR? Do not round intermediate calculations. Round your...
-
What are the skills needed to be an IT auditor? What skills do you have? What weaknesses do you need to build on?
-
Which, if any, of the dichloroethene molecules drawn in Data Table II (3.) (4.) and (5.) are geometric isomers? A. B. C. D. cis-1,2-dichloroethene and trans-1,2-dichloroethene...
-
Figure P.11.49 shows a transparent ring on an otherwise opaque mask. Make a rough sketch of its autocorrelation function, taking l to be the center-to-center separation against which you plot that...
-
Return to Eq. (12.21) and separate it into two terms representing a coherent and an incoherent contribution, the first arising from the superposition of two coherent waves with irradiances of |? ? 12...
-
Referring to the previous two problems with the cosine grating oriented horizontally, make a sketch of the electric-field amplitude along y' with no filtering. Plot the corresponding image irradiance...
-
Simplify the expression. Assume all variables represent positive real numbers. 3)125 k7q8
-
Firms and markets Yossi Spiegel Problem set 2: Technology and cost functions Problem 1 A firm produces a single output using two inputs in fixed proportions. Suppose that it is known that the firm's...
-
Write the equation of the line show in the graph below. 10+ 8 6 4 2 10 -8 -6 4 -2 2 4 6 8 10 +2 N -4 -6 -8 D

Study smarter with the SolutionInn App


