Consider the voltaic cell: a. Determine the direction of electron flow and label the anode and the
Question:
Consider the voltaic cell:
a. Determine the direction of electron flow and label the anode and the cathode.
b. Write a balanced equation for the overall reaction and calculate E°cell.
c. Label each electrode as negative or positive.
d. Indicate the direction of anion and cation flow in the salt bridge.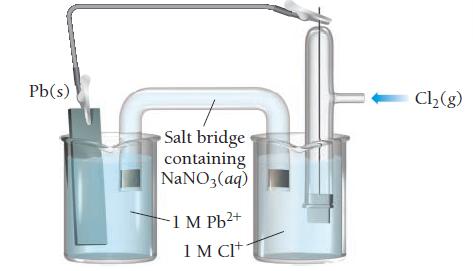
Transcribed Image Text:
Pb(s) Salt bridge containing NaNO3(aq) -1 M Pb²+ 1 M CI+ Cl₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
To answer your questions about the voltaic cell in the image provided Ill go through each point one by one a Determine the direction of electron flow ...View the full answer

Answered By

Lilian Nyambura
Hi, am Lilian Nyambura, With extensive experience in the writing industry, I am the best fit for your writing projects. I am currently pursuing a B.A. in Business Administration. With over 5 years of experience, I can comfortably say I am good in article writing, editing and proofreading, academic writing, resumes and cover letters. I have good command over English grammar, English Basic Skills, English Spelling, English Vocabulary, U.S. English Sentence Structure, U.K. or U.S. English Punctuation and other grammar related topics. Let me help you with all your essays, assignments, projects, dissertations, online exams and other related tasks. Quality is my goal.
4.80+
378+ Reviews
750+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the voltaic cell: a. Determine the direction of electron flow and label the anode and the cathode. b. Write a balanced equation for the overall reaction and calculate E cell . c. Label each...
-
Ray Holt Corporation has retained you as a consultant on accounting policies and procedures. During 2019, the company engaged in a number of treasury stock transactions, having foreseen an...
-
26. Originally from England, Joy received her permanent Canadian resident status three years ago. She lives in Edmonton, where she works as a surgeon major hospital. She travels back to her home...
-
What is the timestep value ? And how do I go about altering from downstream to upstream? The following code solves the advection equation 1 2 3 4 5 6 7 8 9- 10 - 11 12 - 13 - 14 - 15 - 16 - 17 18 19...
-
To the Point Manufacturing Company uses the standard costing method. The companys main product is a fine-quality fountain pen that normally takes 2.5 hours to produce. Normal annual capacity is...
-
How have accounting requirements and practices in the Czech Republic been influenced by European Union requirements?
-
In the 1970s, Special Electric Company brokered the sale of crocidolite asbestos, which is the most toxic form of asbestos, to Johns- Manville Corporation. Special Electric never held possession of...
-
Comm Devices (CD) is a division of Worldwide Communications, Inc. CD produces pagers and other personal communication devices. These devices are sold to other Worldwide divisions, as well as to other...
-
Business Scenario The ABC corporation is a newly established company and the owner is looking for an efficient method of collecting, storing, and manipulating data. The corporation offers a variety...
-
Use line notation to represent each electrochemical cell in Problem 43. Problem 43 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that...
-
Calculate the standard cell potential for each of the electrochemical cells in Problem 44. Problem 44 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the...
-
Ostrich Enterprise is a small production firm that operates in Sweden. The company produces meatballs for canteens. During June 2020, the standard variable overhead has been budgeted as follows:...
-
List 5 passive income subject to Final Tax. Write the applicable final tax rate beside the passive income.
-
Gibson Industries is issuingan11-yearsemi-annualinterest coupon bond witha $1,000 par value and an8%couponrate. Investors are willing to pay $972 for this bond. Gibson is in the 34% tax bracket. What...
-
Harold Buckeye, worked two jobs in 2018. He inadvertently failed to report his income from one of the jobs on his tax return. Both employers provided Harold with a Form W-2 reporting his wages...
-
"What are the distinctions in technique and outcome between the negative staining procedure and the preparation of a positive stain?"
-
The beta of a company is 1.0, the return on the DOW Jones 14%, US treasury bills currently yield 8%, the company has historically maintained a growth rate of 5% in dividends and investors expect to...
-
The demand function for a Christmas music CD is given by x = 0.25(225 p 2 ) Where x (measured in units of a hundred) is the quantity demanded per week and p is the unit price in dollars. a) Evaluate...
-
What recommendations would you make to Big Four firms to help them (1) avoid confrontations with governmental officials in an authoritarian society and (2) deal effectively with such confrontations...
-
Explain how Archimedess principle can be used to measure a persons percentage body fat. Fat has a different density than other body tissue.
-
The specific gravity of a substance is the ratio of its density to the density of water. Among other applications, measurements of the specific gravity are used by mineralogists to determine the...
-
In a neutron star, all the mass has collapsed into a relatively small volume. (a) If a particular neutron star has a radius of 1000 m and a mass of 2.0 10 28 kg, what is its density? (b) Compare...
-
INSTRUCTIONS: One sample activity for a strategic management paper could be a case study analysis. You can choose a real-world company and examine its strategic management practices, analyzing its...
-
Could you elaborate on the theoretical underpinnings of primary keys within the relational model, exploring their role in defining entity integrity, establishing referential integrity constraints,...
-
St. Johns River Shipyards is considering the replacement of an 8-year-old riveting machine with a new one that will increase earnings before depreciation from $27,000 to $46,000 per year. The new...

Study smarter with the SolutionInn App


