Determine the cell potential for an electrochemical cell based on the following two half-reactions: Oxidation: Cu(s)- >>>
Question:
Determine the cell potential for an electrochemical cell based on the following two half-reactions: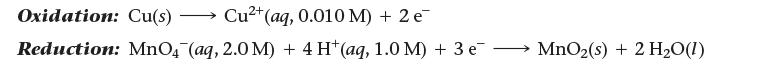
Transcribed Image Text:
Oxidation: Cu(s)- →>>> Cu²+ (aq, 0.010 M) + 2 e Reduction: MnO4 (aq, 2.0 M) + 4 H(aq, 1.0 M) + 3 e MnO₂ (s) + 2 H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Oxidation Anode Reduction Cathode 2MnO4 aq 4Haq 3e Ecell ...View the full answer

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An experimental fuel cell has been designed that uses carbon monoxide as fuel. The overall reaction is 2CO(g) + O2(g) 2CO2(g) The two half cell reactions are CO + O2- CO2 + 2e- O2 + 4e 2O2- The...
-
Determine the cell potential of the following cell. Pb|PbSO4(s), SO42(1.0 M)||H+(1.0 M) |H2(1.0 atm)|Pt
-
Determine the cell potential of the following cell. Pt|H2(1.0 atm)|H+(1.0 M)||Cl(1.0 M), AgCl(s) |Ag
-
You have just been given a $454,000, which you decide to invest at an APR of 6.7 percent. If you were to withdraw $38,500 at the end of each year, starting at the end of this year, how many years...
-
Sevilla Consulting offers environmental consulting services worldwide. The managers of branch offices are rewarded for superior performance with bonuses based on the economic value that the office...
-
With the growing popularity of casual surf print clothing, two recent MBA graduates decided to broaden this casual surf concept to encompass a surf lifestyle for the home. With limited capital, they...
-
What are the three parts of a make rule?
-
Consider a three- firm supply chain consisting of a retailer, manufacturer, and supplier. The retailers demand over an 8- week period was 100 units each of the first 2 weeks, 200 units each of the...
-
Explore Incorporated is a new start - up specializing in renting camper vans. On January 1 , Year 1 , the company purchased a new camper van with a total cost of $ 8 8 , 4 0 0 . The company estimates...
-
In an electrochemical cell, Q = 0.0010 and K = 0.10. What can you conclude about E cell and E cell ? (a) E cell is positive and E cell is negative. (b) E cell is negative and E cell is positive. (c)...
-
A redox reaction has an E cell = -0.56 V. What can you conclude about the equilibrium constant (K) for the reaction? a) K < 1 d) Nothing b) K> 1 c) K = 0 can be concluded about K from Ecell.
-
The following data represent the rates (micrometers per hour) at which a razor cut made in the skin of anesthetized newts is closed by new cells. (a) Can we say that the data are approximately...
-
5. Determine the capacitance required to establish a capacitive reactance that will match that of a 2 mH coil at a frequency of 50 kHz. 6. In Fig. 1, a. What is the sinusoidal expression for the...
-
Could you elaborate on the concept of "biofilm" and elucidate the mechanisms and environments conducive to its formation?
-
Public International Law and International Trade Compare and contrast the effects of multilateral trade arrangements (for example the World Trade Organisation/GATT) and regional trade organisations...
-
Using the information above, prepare abbreviated income statements for the years 2021 and 2022 using the cash basis of accounting. Sales $ Expenses Net Income/(Loss) +A $ Blue Spruce Corp. Income...
-
Complete the Instalment Activity Statement using the following information: An invoice totalling $5,505 did not have an ABN so the business withheld the mandatory amount from the payment to the...
-
Suppose you have the following hypothetical demand or sales function. Qx = -4Px + 2Py + 0.20I + 0.04A And PX = $200, (price of good X) PY = $230,(price of good Y) I = $15,000 (disposable per capita...
-
For the next several days, take notes on your listening performance during at least a half-dozen situations in class, during social activities, and at work, if applicable. Referring to the traits of...
-
Consider the transnational and rotational kinetic energies of a disc that rolls without slipping. Show that the ratio of these energies is independent of the size (the radius) of the wheel.
-
It has been proposed that large flywheels could be used to store energy. Consider a flywheel made of concrete (density 2300 kg/m 3 ) in the shape of a solid disk, with a radius of 10 m and a...
-
You are given two objects and a ramp. Explain how you could measure the ratio of the moments of inertia of the two objects. Assume the two objects are both round (so that both roll), have the same...
-
1.) Use the information in the table below to answer the questions a- e. Debt Common Stock Preferred Stock Market Tax Rate 50,000 bonds with 7.0 % coupon rate, payable annually, $1,000 par value, 12...
-
5. An investor who owns a bond with a 9% coupon rate that pays interest semiannually and matures in three years is considering its sale. If the yield-to-maturity on the bond is 11%, find the price of...
-
6. The table below provides the monthly high temperature in Calgary, Alberta for 2021 (calgary.weatherstats.ca). Month Temperature "C 1 January 9.7 2 February 9.4 3 March 18.7 4 April 21.7 5 May 27.2...

Study smarter with the SolutionInn App


