Find G rxn for the reaction: Use the following reactions with known G rxn values: 3 C(s)
Question:
Find ΔG°rxn for the reaction:![]()
Use the following reactions with known ΔG°rxn values: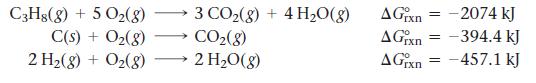
Transcribed Image Text:
3 C(s) + 4H₂(g) → C3H8(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To work this problem you need to manipulate the given reactions with known values of AGxn in such a ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following reaction taking place at 300 K: 2 NH 3 ( g ) + 3 Br 2 ( g ) N 2 ( g ) + 6 HBr ( g ) K c = ?? a) Use the following reactions with known values of K c to determine the value...
-
Proper management and leadership are key when dealing with finances in sports. Explain the difference between the two and how they will apply to the financial structure of your sports organization?
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
In the figure below, a square of edge lengths is formed by four spheres of masses, m, M, m3, and m4. What is the x component and the y component of the net gravitational force from them on a central...
-
Tilly Issac is the assistant controller for Tagwell Corporation, a leading producer of home appliances. Her friend Zack Marsh is the supervisor of the firms Cookware Department. Marsh has the...
-
You have graduated with a business degree, and you have worked for three years for a small management consulting firm. Ivan Steeger (1352 Bull Run Road, Milltown, OR 97111) is a client who has been...
-
The company is committed to ethical conduct and has no tolerance for fraud and unethical behavior. There are some concerns about abuses in your department. Do you know anything about the concerns I...
-
Quigley Corporations trial balance at December 31, 2014, is presented below. All 2014 transactions have been recorded except for the items described below. Unrecorded transactions 1. On January 1,...
-
Pinehurst Company was formed in Year 1 and experienced the following accounting events during the year: 1. Issued common stock for $18,400 cash. 2. Earned cash revenue of $26,100. 3. Paid cash...
-
A reaction is spontaneous under a certain set of conditions. What can you conclude about G rxn and Q? (a) AGxn < 0, Q > K (c) AGrxn > 0, Q < K (b) AGxn <0, Q < K (d) AGxn> 0, Q > K
-
Why does the entropy of a gas increase when it expands into a vacuum?
-
Consider the audit procedure referred to as the search for unrecorded liabilities. a. How does the auditor perform it? b. When does the auditor perform it? c. What is its purpose? d. Describe the...
-
Identify one Strength in K & N's approach to the workforce. Base your response on the guidelines in the Team Project Instructions for writing a Strength comment: Begin by identifying the diagnostic...
-
In a single Word document, minimum 7 full pages (not including cover page and citations), using appropriate APA, answer the following questions using the week's reading materials and video to guide...
-
How does a strong mission and vision statement for a company influence the organizations strategic decision making? Select a company from the hospitality industry that you think has a strong mission...
-
Briefly describe what you would do to back up your home computer to protect yourself from a computer failure. What are the most common cause of computer failures? How would your procedures protect...
-
Regarding The new Simply Orange Juice lawsuit 2023: Who will this action have an effect on? What social problems would the action affect? What aspect of the environment would the action affect? Will...
-
Although Congress established the Foreign-Trade Zones in 1934 to encourage U.S. firms to participate in global trade, it is still a viable tool today in continuing trade.
-
In the busy port of Chennai, India, the number of containers loaded onto ships during a 15-week period is as follows: 1. Develop a linear trend equation to forecast container loadings. 2. Using the...
-
Sound waves in water travel at a speed of approximately 1500 m/s. What is the wavelength of a sound wave that has a frequency of 440 Hz?
-
The neck of a guitar is designed with frets as shown in Figure Q12.8. A player can hold a string against one of the frets and thus shorten the vibrating length L of the string. In this way, a...
-
A wave on a Slinky has a frequency of 2.3 Hz. It takes 3.5 s to travel the length of the Slinky (0.30 m). What is the wavelength?
-
ChiBoxer Limited has risky assets-in-place with values next year presented as follows: Assets-in-place Interest Rate Probability Low 1/3 $100 million High 2/3 $10 million All other relevant public...
-
1: Managing Repair Operations Fuel processing units arrive at your facility for repair at a steady rate of 3 units at the beginning of each week. At the start of each week, you decide how many units...
-
Perform a 5 year financial analysis of expanding beds by 50% Group 2 - attach an xls showing the impact of adding operations to Saturday. Group 3 - attach an xls showing the impact of adding 50%...

Study smarter with the SolutionInn App


