Find the equilibrium constant at 298 K for the reaction. The K f for [Cu(NH 3 )
Question:
Find the equilibrium constant at 298 K for the reaction.![]()
The Kf for [Cu(NH3)2]+ = 6.3 * 1010 and the rest of the data needed are in Appendix II.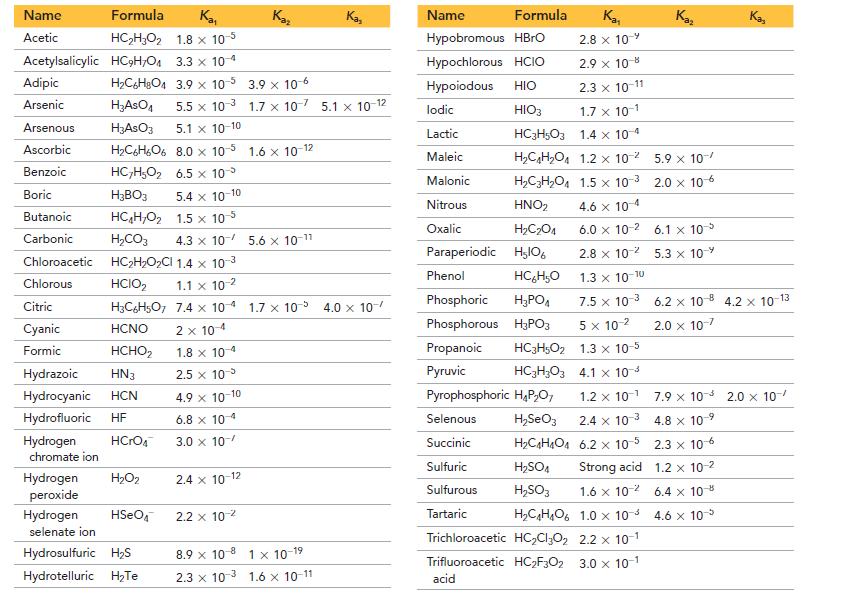
Transcribed Image Text:
2 [Cu(NH3)2](aq) = [Cu(NH3)4]²+ (aq) + Cu(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To find the equilibrium constant for the reaction 2 CuNH32aq CuNH34 aq Cus at 298 K we ca...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For the following exercises, use the vertical line test to determine which graphs show relations that are function x m II
-
Find the equilibrium constant at 298 K for the reaction. The K f for [Cu(CN) 2 ]- = 1.0 * 10 24 and the rest of the data needed are in Appendix II. [Ag(CN)] (aq) + Cu(s) [Cu(CN)] (aq) + Ag(s)
-
Muharraq Co. paid the following different costs in 2020: 1. $2,000,000 to acquire a machine to be used in the R&D projects. The machine has a 4- year useful life (Muharraq Co. started using the...
-
If f(x) = 5x + 1, find and simplify each of the following in Problem. f(f( -1))
-
Jigar Tech, Inc., is authorized to issue $1,800,000 in bonds on June 1. The bonds carry a face interest rate of 9 percent, which is to be paid on June 1 and December 1. Prepare entries in journal...
-
Your sister operates Emigrant Parts Company, an online boat parts distributorship that is in its third year of operation. The income statement is shown at the top of the following page and was...
-
Jerome M. Eisenberg is an antiquities dealer and a self-proclaimed expert in classical antiquities with a doctorate in Roman, Egyptian, and Near Eastern art. Maurice E. Hall Jr. is an art dealer who...
-
Avaya Corporation had the following stock outstanding from 2011 through 2014: Preferred stock: $100 par value, 8 percent cumulative, 5,000 shares authorized, issued, and outstanding Common stock: $10...
-
3 3. 7 of the coins in a box are nickels. The rest are dimes. If there are 24 dimes, how many nickels are there?
-
Tin exists in two allotropic forms. Gray tin has a diamond structure, and white tin has a close-packed structure. Predict which allotrope is (a) Denser, (b) A conductor of electricity. Predict the...
-
Hydrogen can be in both the octahedral and tetrahedral holes for lanthanum. Determine the percentage of the holes that are filled if the formula is LaH 2.76 .
-
When obligation to pay is binding on account of a past event, it is recognized as (a) Contingent liability (b) Liability at historical cost (c) Liability at fair value (d) None of these
-
The Family Fine Arts Center charges $22 per adult and $13 per senior citizen for its performances. On a recent weekend evening when 482 people paid admission, the total receipts were $7283. How many...
-
Indicate how Managerial Accounting can help you make better investment decisions based on numbers.
-
The Fed has been raising interest rates recently, and you believe that it will continue to do so for at least the next year. You also believe that the Fed's actions will raise interest rates broadly...
-
A cruise ship needs to book at least 2,052 passengers to be profitable, but the most passengers the ship can accommodate is 2,462. Model the numbers of passengers that need to be booked to ensure the...
-
How does Chinua Achebe challenge colonialist perspectives in "Things Fall Apart," offering a nuanced portrayal of Igbo culture and the impact of European colonization on traditional African societies?
-
Suppose that a person won the Florida lottery and was offered a choice of two prizes: (1) $500,000 or (2) a coin-toss gamble in which he or she would get $1 million if a head were flipped and zero...
-
How does health insurance risk differ from other types of insurance risk (e.g., automobile or homeowners insurance)? What is the difference between cost sharing and cost shifting? Is retiree health...
-
Two point particles of charge Q 1 = 45 C and Q 2 = 85 C are found to have a potential energy of 40 J. What is the distance between the charges?
-
Two particles with Q 1 = 45 C and Q 2 = 85 C are initially separated by a distance of 2.5 m and then brought closer together so that the final separation is 1.5 m. What is the change in the electric...
-
The nucleus of a helium atom contains two protons. In a simple model of this nucleus, the protons are viewed as point particles separated by 1.0 fm (1.0 10 -15 m). What is the electric potential...
-
5. An integer n with at least 4 digits is called happy if it has the following four properties. All of its digits are nonzero. For every set of four consecutive digits of n, the four-digit integer...
-
4. Determine the largest positive integer n with the property that if x, y, and z are integers satisfying 3x=5y = 7z, then xyz is a multiple of n.
-
Crow Brothers, a real estate developer, builds houses in three states. The projected number of units of each model to be built in each state is given by the matrix A below: [60 80 A= 20 30 l10 15 120...

Study smarter with the SolutionInn App


