H 2 reacts with the halogens (X 2 ) according to the reaction: where X 2 can
Question:
H2 reacts with the halogens (X2) according to the reaction:![]()
where X2 can be Cl2, Br2, or I2. Use the thermodynamic data in Appendix IIB to calculate ΔH°, ΔS°, ΔG°, and Kp for the reaction between hydrogen and each of the three halogens. Which reaction is most spontaneous? Least spontaneous? What is the main factor responsible for the difference in the spontaneity of the three reactions? Does higher temperature make the reactions more spontaneous or less spontaneous?
Appendix IIB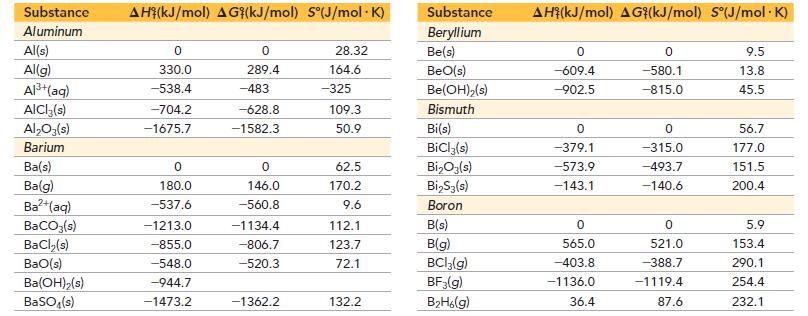
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





