Identify the Lewis acid and Lewis base from among the reactants in each equation. a. Ag (aq)
Question:
Identify the Lewis acid and Lewis base from among the reactants in each equation.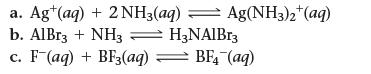
Transcribed Image Text:
a. Ag (aq) + 2NH3(aq) → Ag(NH3)2+(aq) b. AlBr3 + NH3 H3NAIBr3 C. F (aq) + BF3(aq) = BF4 (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a Agg 2NH39 AgNH32aq Lewis acid Lewis base b AIBT33 Lewis aci...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the Lewis acid and Lewis base among the reactants in each of the following reactions: (lq denotes liquid ammonia as solvent)
-
Identify the Lewis acid and Lewis base from among the reactants in each equation. 3+ a. Fe+ (aq) + 6 HO(1) 2+ b. Zn+ (aq) + 4 NH3(aq) + BF3(g) c. (CH3)3N(g) Fe(HO)6+ (aq) 2+ = Zn(NH3)4+ (aq)...
-
Designate the Lewis acid and Lewis base in each of the following reactions: (a) (b) (c) CI CH,CH2 CIAIC CH OHBFCH3 CH3 CHs CH
-
At the beginning of the year, Anna began a calendar-year business and placed in service the following assets during the year: Asset Date Acquired Cost Basis Computers 1/30 $28,000 Office desks 2/15...
-
The direct labor rate variance is never controllable. Do you agree or disagree? Why?
-
As the driver steps on the gas pedal, a car of mass 1 160 kg accelerates from rest. During the first few seconds of motion, the cars acceleration increases with time according to the expression a =...
-
What is meant by inductive bias? Is it a good thing? What is the inductive bias of the ID3 algorithm?
-
Hatcher Enterprises uses a chemical called Rbase in production operations at five divisions. Only six suppliers of Rbase meet Hatchers quality control standards. All six suppliers can produce Rbase...
-
The elastic (or spring) potential energy associated with the spring force is given by 1 PE ==kx2 S What does the "x" refer to in the above equation? kx 3
-
Based on these molecular views, determine whether each pictured acid is weak or strong. H3O+- HF- F (a) CHO H3O+- HCHO2- (c) HO+- (b) I- NO3- HO+ (d)
-
Classify each species as either a Lewis acid or a Lewis base. a. BeCl b. OH- C. B(OH)3 d. CN-
-
The output in Figure 18.9 from applying Holt-Winters exponential smoothing to data, using "optimal" smoothing constants. a. The value of zero that is obtained for the trend smoothing constant means...
-
1. Prove that two colliding particles cannot transform into a single photon. 2 . Explain why a photon that strikes a free electron cannot be absorbed: ???? + ???? ???? . Such a reaction does take...
-
An escalator is used to move 54 people (60kg each) per minute from the first floor of a departm store to the second floor, 5m above. The power of the escalator's motor required should be at le a....
-
4.0g bullet is fired from a 5.0kg gun with a speed of 600 m/sec. What is the speed of the recoil of the gun? What is the direction of the recoil?
-
(A) A large balloon used to sample the upper atmosphere is filled with 565 m 3 of helium. What is the mass of the helium (in kg)? (B) Find the gauge pressure (in psi) at the bottom of a freshwater...
-
The current temperature of the Earth is estimated to be approximately 300 Kelvin. Assume for some natural reason (i.e. an asteroid impact or major volcanic eruption), the temperature of the Earth...
-
Compute increases (decreases) in percents for both Years 6 and 7 by entering all the missing data in the table below. Analyze and interpret any significant results revealed from this trendanalysis....
-
In Problem 8.43, determine the smallest value of for which the rod will not fall out of the pipe. IA -3 in.-
-
Suppose the density of the Earth was somehow reduced from its actual value to 1000 kg/m 3 (the density of water). Find the value of g, the acceleration due to gravity, on this new planet. Assume the...
-
Calculate the acceleration due to gravity on the surface of Mars.
-
Find the gravitational force of the Sun on the Earth.
-
1. A lab instructor is observing placement of a Foley catheter by a senior nursing student. If the student is in the active experimentation phase of Kolbs Theory of Experiential Learning, what action...
-
1. A nursing student is learning about effective time management in her first semester of nursing school. Which action by the student indicates that she understands the first critical step? a.Setting...
-
1.The nurse understands that there are four key habits for managing the work of success. Which action by the nurse demonstrates her understanding? a.Participating in a yoga clasNs b.Analyzing case...

Study smarter with the SolutionInn App


