Imagine that a molecule with six electron groups is confined to two dimensions and therefore has a
Question:
Imagine that a molecule with six electron groups is confined to two dimensions and therefore has a hexagonal planar electron geometry. If two of the six groups are lone pairs, where are they located?
(a) Positions 1 and 2
(b) Positions 1 and 3
(c) Positions 1 and 4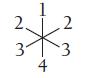
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





