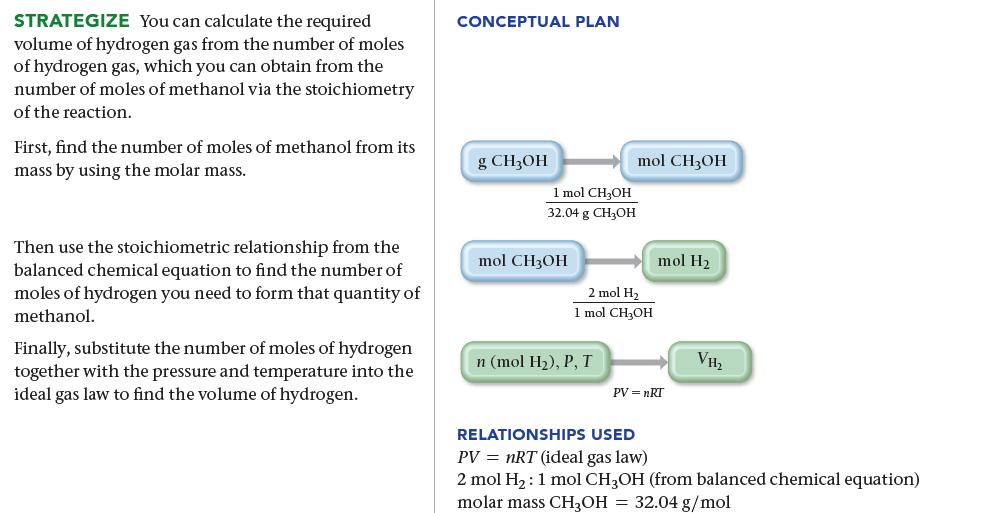
Methanol (CH 3 OH) can be synthesized by the reaction: What volume (in liters) of hydrogen gas,
Question:
Methanol (CH3OH) can be synthesized by the reaction:![]()
What volume (in liters) of hydrogen gas, at a temperature of 355 K and a pressure of 738 mmHg, is needed to synthesize 35.7 g of methanol?
Transcribed Image Text:
CO(g) + 2 H₂(8) CH3OH(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
357 g CH3OH X 11142 mol CH3OH X VH VH HRT P ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
CH 3 OH can be synthesized by the reaction: What volume of H 2 gas (in L), at 748 mmHg and 86 C, is required to synthesize 25.8 g CH 3 OH? How many liters of CO gas, measured under the same...
-
Methanol (CH 3 OH) can be synthesized by the reaction: CO(g) + 2 H 2 (g) CH 3 OH(g) What volume (in liters) of methanol gas, measured at a temperature of 473 K and a pressure of 820 mmHg, is...
-
Consider the chemical reaction: How many liters of hydrogen gas are formed from the complete reaction of 15.7 g C? Assume that the hydrogen gas is collected at a pressure of 1.0 atm and a temperature...
-
Question: If you were a consultant and for the below M&A case, what questions would you ask as a consultant of the acquiring company of the mine and in order to complete the valuation: A firm is...
-
What are Internet firewalls and proxy servers? How are they created? How do businesses use them for Internet security?
-
Use + and - notation to show the dispersal of charge to the hydrogens in NH4+ and BH4-
-
Briefly explain the manner in which (a) input and output controls and (b) processing controls may be tested when an EDP system is used for cash receipts transactions.
-
Rouse Chemical has spent $ 241,000 to refine 71,000 gallons of acetone, which can be sold for $ 1.90 a gallon. Alternatively, Rouse Chemical can process the acetone further. This processing will...
-
Presented below is information related to the Annandale Division of Lumber, Inc. Contribution margin $ 1 , 2 1 1 , 9 0 0 Controllable margin $ 8 9 5 , 1 8 0 Average operating assets $ 4 , 0 6 9 , 0 0...
-
Which gas has the greatest kinetic energy at STP? a) He b) Ne c) Ar d) None of the above (All have the same kinetic energy.)
-
What is the ideal gas law? Why is it useful?
-
Using separate feeds of A and B sketch the contacting pattern and reactor conditions which would best promote the formation of product R for the following systems of elementary reactions A + B R 2A ...
-
A municipality, as owner, retained the services of an architect to design a new police station. The architect has entered into a contract with an engineering firm to provide structural design...
-
Wright, Bell, and Edison are partners and share income in a 2:5:3 ratio (in ratio form: Wright, 2/10; Bell, 5/10; Edison 3/10). The partnerships capital balances are as follows: Wright, $33,000, Bell...
-
Find deflection a point c using double integration method? E=200GPa, I=95x10 6 mm 4 49 KHM 23kW 2174/m A 3 1.7m 46KN
-
Dats Systems has stock currently priced at $50.00 per share. If investors are earning a 7% return on Dats Systems, what is the company's annual dividend payment per share?
-
Your client, Ms. Kimberly Hall, stands convicted under your state law for charges involving theft, trafficking in stolen property, fraud, and alteration of vehicle identification numbers. Hall runs a...
-
Review the information presented in the chapter in the discussion of swap transactions between Global Crossing and Qwest. a. What type of fraud did the companies commit? b. Consider the fraud...
-
Quality Chicken grows and processes chickens. Each chicken is disassembled into five main parts. Information pertaining to production in July 2012 is: Joint cost of production in July 2012 was $50. A...
-
Determine the moment at B, then draw the moment diagram for each member of the frame. Assume the support at A is fixed and C is pinned. EI is constant. 2 kN/m A -3 m- 4 m
-
Determine the moments at Band D, then draw the moment diagram. Assume A and C are pinned and B and D are fixed connected. EI is constant. 8k -10 ft- 10 ft- -15 ft- 12 ft
-
Determine the moment that each member exerts on the joint at B, then draw the moment diagram for each member of the frame. Assume the support at A is fixed and C is a pin. EI is constant. 2 k/ft 6 ft...
-
Jason is a 15-year-old boy, born out of wedlock. His mother, Jane, lost both her parents in a car accident at the age of 10 years. At the time of their untimely death, they were staying in a flat in...
-
Indigo Associates Inc. reports the following account balances for the year ending June 30, 2025: Accounts payable $22,000 Accounts receivable 38,000 Cash and cash equivalents 16,500 Goodwill 129,000...
-
1. (5 pts) Given the following recurrence relation, { B(n) B(1)=5 B(n-1)+5 for n 2 write down the corresponding recursive algorithm (pseudocode or in any program language).

Study smarter with the SolutionInn App


