Methanol (CH 3 OH) has been suggested as a fuel to replace gasoline. Write a balanced equation
Question:
Methanol (CH3OH) has been suggested as a fuel to replace gasoline.
Write a balanced equation for the combustion of methanol, find ΔH°rxn, and determine the mass of carbon dioxide emitted per kJ of heat produced. Use the information from the previous exercise to calculate the same quantity for octane, C8H18. How does methanol compare to octane with respect to global warming?
Previous Exercise
Determine the mass of CO2 produced by burning enough of each fuel to produce 1.00 * 102 kJ of heat. Which fuel contributes least to global warming per kJ of heat produced?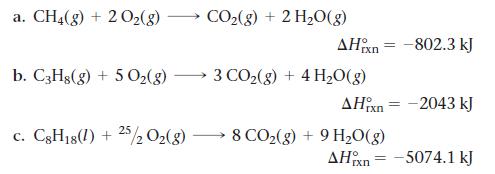
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





