Nitric oxide reacts with chlorine gas according to the reaction: A reaction mixture initially contains equal partial
Question:
Nitric oxide reacts with chlorine gas according to the reaction: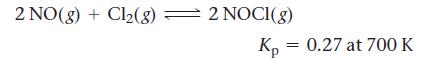
A reaction mixture initially contains equal partial pressures of NO and Cl2. At equilibrium, the partial pressure of NOCl is 115 torr. What were the initial partial pressures of NO and Cl2?
Transcribed Image Text:
2 NO(g) + Cl₂(8) = 2 NOCI(g) Kp 0.27 at 700 K =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
P NO...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Elemental phosphorus reacts with chlorine gas according to the equation: A reaction mixture initially contains 45.69 g P 4 and 131.3 g Cl 2 . Once the reaction has occurred as completely as possible,...
-
Pure nitrosyl chloride (NOCl) gas was heated to 240°C in a 1.00-L container. At equilibrium the total pressure was 1.00 atm and the NOCl pressure was 0.64 atm. (a) Calculate the partial pressures...
-
Aluminum reacts with chlorine gas according to the following equation shown below. How many moles of Cl 2 are required to react with 0.11 mol of Al? 2 Al( s ) + 3 Cl 2 ( g ) 2 AlCl 3 ( s )
-
Find the amplitude, period, and phase shift of function. Graph function. Show at least two periods. 2 3 - cos ( 6)
-
Stewart Fibers, Inc., specializes in the manufacture of synthetic fibers that the company uses in many products such as blankets, coats, and uniforms for police and firefighters. Stewart has been in...
-
A liquid containing 60 mol% toluene and 40 mol% benzene is continuously distilled in a single-equilibrium-stage unit at atmospheric pressure. What percent of benzene in the feed leaves in the vapor...
-
Jack DeCoster owned Quality Egg, LLC, an Iowa egg production company. Jacks son, Peter DeCoster, served as the companys chief operating officer. Jack also owned and operated several egg production...
-
Vulcan Flyovers offers scenic overflights of Mount St. Helens, the volcano in Washington State that explosively erupted in 1982. Data concerning the companys operations in July appear below: The...
-
Create a relational model for the following ERD. Please do not develop a graphical version of the relational databases. Please just list the tables, attributes, primary keys and foreign keys. Dept...
-
A reaction vessel at 27 C contains a mixture of SO 2 (P = 3.00 atm) and O 2 (P = 1.00 atm). When a catalyst is added, this reaction takes place: 2 SO 2 (g) + O 2 (g) 2 SO 3 (g). At equilibrium, the...
-
The system described by the reaction CO(g) + Cl 2 (g) COCl 2 (g) is at equilibrium at a given temperature when PCO = 0.30 atm, PCl 2 = 0.10 atm, and PCOCl 2 = 0.60 atm. An additional pressure of Cl...
-
In the figure, OP is a radius and PQ is tangent to circle O. If the radius of circle O is 10 and 16 = QR, what is the length of PQ? A) 16 B) 20 C) 24 D) 28 O 10 P R 16 Q
-
A2A SpA is an Italian utility firm. Its most recent dividend was 0.013 per share. In the past year, the company has experienced financial difficulties and the share price has dropped by more than 20...
-
An investment project has annual cash inflows of 20,000, 35,400, 48,000 and 54,500, and a discount rate of 14 per cent. What is the discounted payback period for these cash flows if the initial cost...
-
Consider four different equities, all of which have a required return of 15 per cent and a most recent dividend of 4.00 per share. Equities W, X and Y are expected to maintain constant growth rates...
-
What are the main elements of corporate finance? How might these elements relate to typical family life?
-
What is the profitability index, and how is it calculated? Discuss the main applications of the profitability index in capital budgeting. When is it most useful and what are its weaknesses? What is...
-
Refer to the information in QS 20-2 and QS 20-3. Blanches Boxes requisitioned $ 9,000 of indirect materials from its raw materials and used $ 10,000 of indirect labor in its production of boxes....
-
Why did management adopt the new plan even though it provides a smaller expected number of exposures than the original plan recommended by the original linear programming model?
-
For the data in Problem 10.22, compute the energy loss for gradual contractions with each of the cone angles listed in Figs. 10.10 and 10.11. Plot energy loss versus the cone angle. Figure 10.10...
-
Determine the energy loss for a gradual contraction from a 4-in Schedule 80 steel pipe to a 1-in Schedule 80 pipe for a flow rate of 250 gal/min. The cone angle for the contraction is 76.
-
Determine the energy loss for a sudden contraction from a 4-in Schedule 80 steel pipe to a 1-in Schedule 80 pipe for a flow rate of 250 gal/min.
-
Describe the organizational structure of the Microsoft company . Do you think it is optimally organized to achieve its goals? Why or why not?
-
Explain how changing economic and competitive pressure have had an impact on the organization in which you are working or one in which you have worked. How has your business responded to this...
-
e. Accrued Salaries Expense at December 31, 2023 Requirements $350 1. Open T-accounts using the balances in the unadjusted trial balance. 2. Prepare the adjusting entries and post to the T-accounts....

Study smarter with the SolutionInn App


