Silver sulfate dissolves in water according to the reaction: A 1.5-L solution contains 6.55 g of dissolved
Question:
Silver sulfate dissolves in water according to the reaction: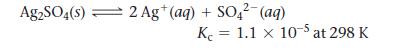
A 1.5-L solution contains 6.55 g of dissolved silver sulfate. If additional solid silver sulfate is added to the solution, will it dissolve?
Transcribed Image Text:
Ag2SO4(s) 2 Ag+ (aq) + SO42- (aq) K 1.1 x 10-5 at 298 K =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Additi...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
A sample consisting of 22.7 g of a nongaseous, unstable compound X is placed inside a metal cylinder with a radius of 8.00 cm, and a piston is carefully placed on the surface of the compound so that,...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
To the right is the graph of the position of the object versus time: Which of graphs below correctly shows the object's velocity versus time? velocity (m/s) velocity (m/s) 1.5 1 0.5 -0.5 -1 -1.5 15...
-
Jonfran Company manufactures three different models of paper shredders including the waste container, which serves as the base. While the shredder heads are different for all three models, the waste...
-
A block is placed on a plane inclined at angle . The coefficient of friction between the block and the plane is = tan . The block is given a kick so that it initially moves with speed V horizontally...
-
CMS is a claims processing company in Mobile, Alabama. Chastity Jones, a black woman, completed an online employment application for a customer service position with CMS. Jones interviewed with a...
-
Southwest Milling Co. purchased a front-end loader to move stacks of lumber. The loader had a list price of $140,000. The seller agreed to allow a 4 percent discount because Southwest Milling paid...
-
Q5). Given the graph y= f(x), graph y=[-2(x-1)]-2 on the same grid on your right. Use at least 4 points: Show steps
-
Nitrogen dioxide dimerizes according to the reaction: A 2.25-L container contains 0.055 mol of NO 2 and 0.082 mol of N 2 O 4 at 298 K. Is the reaction at equilibrium? If not, in what direction will...
-
Consider the reaction: A reaction mixture contains 0.112 atm of H 2 , 0.055 atm of S 2 , and 0.445 atm of H 2 S. Is the reaction mixture at equilibrium? If not, in what direction will the reaction...
-
Refer to Data Set 33 Disney World Wait Times in Appendix B and use the 10 AM wait times for Space Mountain, Rock n Roller Coaster, Tower of Terror, and Flight of Passage. Use a 0.05 significance...
-
Which quality control tool is sometimes referred to as a fishbone diagram because it places a problem statement at the head of the fishbone and uses each big bone in the fishs skeleton as a category...
-
Two aspects of quality policy guide the arboretum development project. One is the Townships Greenspace Policy and the other is Arbnets arboretum certification requirements. While Level One arboretum...
-
What difficulties does an organization face when seeking to change its business model? In your opinion, why have Barnes & Noble and Kodak struggled to shift their business models?
-
Which of the three competitive advantages do you think companies are most willing to outsource for? List any examples you can think of.
-
What three types of variation should one look for in a Run Chart?
-
Refer to the information in Problem 23-3A. Phoenix Companys actual income statement for 2013 follows. Required 1. Prepare a flexible budget performance report for 2013. Analysis Component 2. Analyze...
-
A spacecraft has left the earth and is moving toward Mars. An observer on the earth finds that, relative to measurements made when the spacecraft was at rest, its a. length is shorter b. KE is less...
-
A bartender slides a mug of root beer with mass m = 2.6 kg down a bar top of length L = 2.0 m to an inattentive patron who lets the mug fall a height h = 1.1 m to the floor. The bar top (Fig. P4.72)...
-
Consider once again the swimmer in Example 4.7. Assume she can swim at a velocity of 0.30 m/s and the river is 15 m wide. She needs to get across the river as quickly as possible. (a) What direction...
-
You are a serious basketball player and want to use physics to improve your free-throw shooting. Do an approximate calculation of the minimum speed the ball must have to travel from your hand to the...
-
The salespeople at Sunland, a notebook manufacturer, commonly pressured operations managers to keep costs down so the company could give bigger discounts to large customers. Michael, the operations...
-
Prepare the journal entries required on December 31, 2028. (Credit account titles are automatically indented when the amount is entered. Do not indent manually. If no entry is required, select "No...
-
A company that uses job order costing purchases $52,000 in raw materials for cash. It then uses $20,000 of raw materials as indirect materials and uses $25,400 of raw materials as direct materials....

Study smarter with the SolutionInn App


