Sulfur dioxide is a pollutant emitted primarily by coal-burning power plants and industrial smelters. Sulfur dioxide in
Question:
Sulfur dioxide is a pollutant emitted primarily by coal-burning power plants and industrial smelters. Sulfur dioxide in air affects the respiratory system in humans and is the main cause of acid rain. Thanks to the Clean Air Act and its amendments, sulfur dioxide levels in the United States have dramatically fallen over the last 30 years. The graph below shows the mean sulfur dioxide levels from 136 measuring sites in the United States for the period 1990 to 2016. Examine the graph and answer the questions that follow.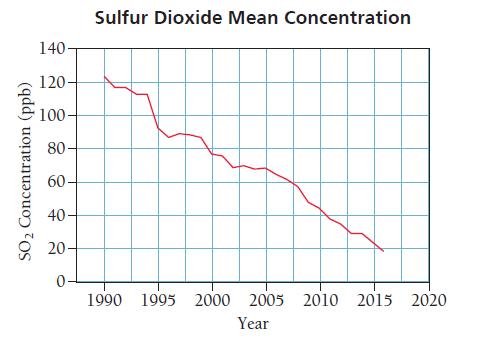
a. On its website, the EPA claims that sulfur dioxide levels have fallen by 85% between 1990 and 2016. Is this claim accurate?
b. The EPA air quality standard for SO2 is 75 ppm. In what year did the average U.S. SO2 concentration begin to meet this standard?
c. What is the percent by mass of S in SO2?
d. A 100 m3 room with an SO2 concentration of 75 ppb contains about 0.021 g SO2. How many sulfur atoms does it contain?
Step by Step Answer:






