Sunscreen contains compounds that absorb ultraviolet light. When sunscreen is applied to skin, it prevents ultraviolet light
Question:
Sunscreen contains compounds that absorb ultraviolet light. When sunscreen is applied to skin, it prevents ultraviolet light from reaching the skin. The graph that follows shows the absorbance of light as a function of wavelength for two different compounds (2-EHMC and TDSA) common in sunscreen. Absorbance is a measure of the amount of light absorbed by the compound— the higher the absorbance, the more light is absorbed. Study the graph and answer the questions.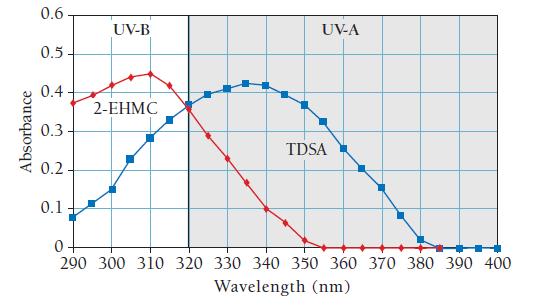
a. Calculate the energy of a photon at the maximum absorption of TDSA.
b. Calculate the energy of a photon at the maximum absorption of 2-EHMC.
c. Which compound absorbs more energy at its maximum absorption?
d. Why do you think sunscreens commonly contain both of these compounds and not just one of them?
e. Assuming that sunlight produces![]()
and that the skin absorbs one-half of these photons (and reflects the other half) calculate the total uv energy absorbed over 0.42 m2 of skin that is exposed to sunlight for one hour. Assume that the average wavelength of the uv photons is 330 nm.
Step by Step Answer:






