The accompanying graph shows the concentration of a reactant as a function of time for two different
Question:
The accompanying graph shows the concentration of a reactant as a function of time for two different reactions. One of the reactions is first order, and the other is second order. Which of the two reactions is first order? Second order? How would you change each plot to make it linear?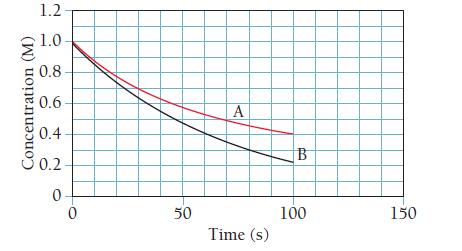
Transcribed Image Text:
Concentration (M) 1.2 1.0 0.8 0.6 0.4 0.2 0 0 50 A Time (s) B 100 150
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
B is first order and A is ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1.9- Martin purchased a van for 12,000 during his six-month period of account to 31 July 2023. What is the maximum amount of capital allowances available for the van for the six months ended 31 July...
-
The gas mileage of a subcompact car is given by m = -0.04x 2 + 3.6x - 49, where x = the speed in miles per hour and m is given in miles per gallon. a) What is the gas mileage for a speed of 25 miles...
-
Holmes Corporation is a leading designer and manufacturer of material handling and processing equipment for heavy industry in the United States and abroad. Its sales have more than doubled, and its...
-
Write a static method max3() that takes three int arguments and returns the value of the largest one. Add an overloaded function that does the same thing with three double values.
-
Peaches and Cream Corporation manufactures cosmetic products that are sold through a network of sales agents. The agents are paid a commission of 16.25% of sales. The income statement for the year...
-
Presented on page 1032 are two independent situations. Situation 1 Hatcher Cosmetics acquired 10% of the 200,000 shares of common stock of Ramirez Fashion at a total cost of $14 per share on March...
-
In the 1970s, Special Electric Company brokered the sale of crocidolite asbestos, which is the most toxic form of asbestos, to Johns- Manville Corporation. Special Electric never held possession of...
-
Agassi Company uses a job order cost system in each of its three manufacturing departments. Manufacturing overhead is applied to jobs on the basis of direct labor cost in Department D, direct labor...
-
3. Market demand is given by: 100-Q, P(Q) = if Q <100 otherwise Suppose there are two firms both with constant marginal cost 50 and no fixed cost and capacity constraint. Assume that firms produce...
-
A particular reaction, A products, has a rate that slows down as the reaction proceeds. The half-life of the reaction is found to depend on the initial concentration of A. Determine whether each...
-
Three different reactions involve a single reactant converting to products. Reaction A has a half-life that is independent of the initial concentration of the reactant, reaction B has a half-life...
-
A hospital pharmacy received ampules of a commonly stocked drug contained in a pink solution. Previously the drug had always been in a clear solution. The pharmacist dispensed the drug for IV...
-
A bank has capital of $200 and a leverage ratio of 5. If the value of the banks assets declines by 10 percent, then its capital will be reduced to a. $100. b. $150. c. $180. d. $185.
-
What are the ways to sustain competitive advantage?
-
If the Fed wants to increase the money supply, it can a. raise income tax rates. b. reduce income tax rates. c. buy bonds in open-market operations. d. sell bonds in open-market operations.
-
Over the past century, ________ has experienced particularly strong growth, and ________ has experienced particularly weak growth. a. China; the United Kingdom b. China; Canada c. the United Kingdom;...
-
According to the theory of liquidity preference, an economys interest rate adjusts a. to balance the supply and demand for loanable funds. b. to balance the supply and demand for money. c....
-
On January 1, 2013, Stone Company issued bonds with a face value of $400,000, a stated rateof interest of 7 percent, and a 10-year term to maturity. Interest is payable in cash on December 31 of each...
-
In Exercises delete part of the domain so that the function that remains is one-to-one. Find the inverse function of the remaining function and give the domain of the inverse function. f(x) = 16x4 -3...
-
The channel shown in Fig. 14.22 has a surface of floatfinished concrete and is laid on a slope that falls 0.1 m per 100 m of length. Calculate the normal discharge and the Froude number for a depth...
-
A rectangular channel must carry 2.0 m 3 /s of water from a water-cooled refrigeration condenser to a cooling pond. The available slope is 75 mm over a distance of 50 m. The maximum depth of flow is...
-
Calculate the depth of flow in a trapezoidal channel with a bottom width of 3 m and whose walls slope 40 with the horizontal. The channel is made of unfinished concrete and is laid on a 0.1-percent...
-
. Use Taylor theorem to derive the error term for the approximate formula f'(x) 1 (3f(x)+4f(x+ h) f(x+2h)). 2h
-
4) Find the area of the following trapezoid. (Special note: This image uses some variables that are slightly different from the formula we developed. So "B" and "b" are just different names for b,...
-
Given the weighted graph below, we will apply Kruskal's algorithm to find a minimal spanning tree. a) The first edge to be chosen will be (Write the edge by giving the two endpoints: e.g. AB, DC,...

Study smarter with the SolutionInn App


