The accompanying graphs show the first ionization energies and electron affinities of the period 3 elements. Refer
Question:
The accompanying graphs show the first ionization energies and electron affinities of the period 3 elements. Refer to the graphs to answer the questions that follow.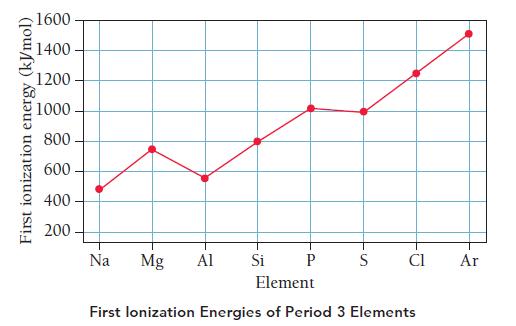
a. Describe the general trend in period 3 first ionization energies as you move from left to right across the periodic table.
Explain why this trend occurs.
b. The trend in first ionization energy has two exceptions: one at Al and another at S. Explain why the first ionization energy of Al is lower than that of Mg and why the first ionization of S is less than that of P.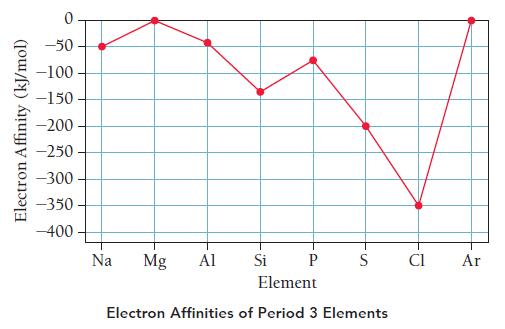
c. Describe the general trend in period 3 electron affinities as you move from left to right across the periodic table. Explain why this trend occurs.
d. The trend in electron affinities has exceptions at Mg and P.
Explain why the electron affinity of Mg is more positive (less exothermic) than that of Na and why the electron affinity of P is more positive (less exothermic) that that of Si.
e. Determine the overall energy change for removing one electron from Na and adding that electron to Cl. Is the exchange of the electron exothermic or endothermic?
Step by Step Answer:






