The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon
Question:
The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon dioxide). The carbon dioxide on Venus might be in equilibrium with carbonate ions in minerals on the planet’s crust. Two possible equilibrium systems involve CaSiO3 and MgSiO3: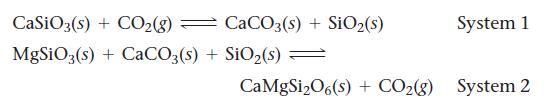
The first graph shows the expected pressures of carbon dioxide (in atm) at different temperatures for each of these equilibrium systems. (Note that both axes on this graph are logarithmic.)
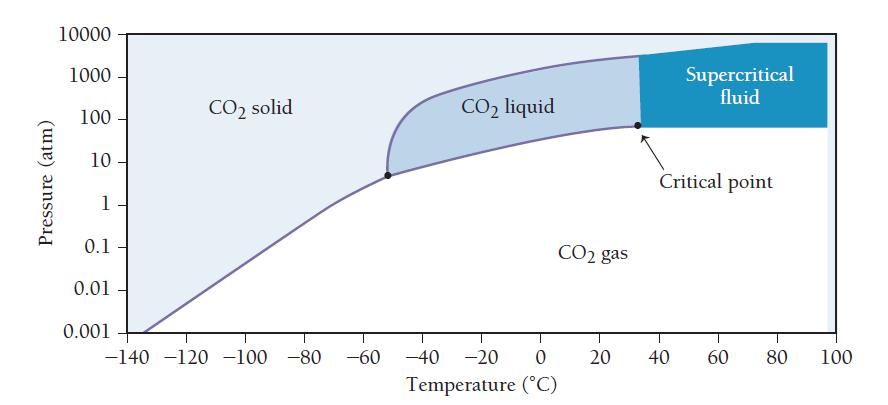
The second graph is a phase diagram for carbon dioxide. Examine the graphs and answer the questions.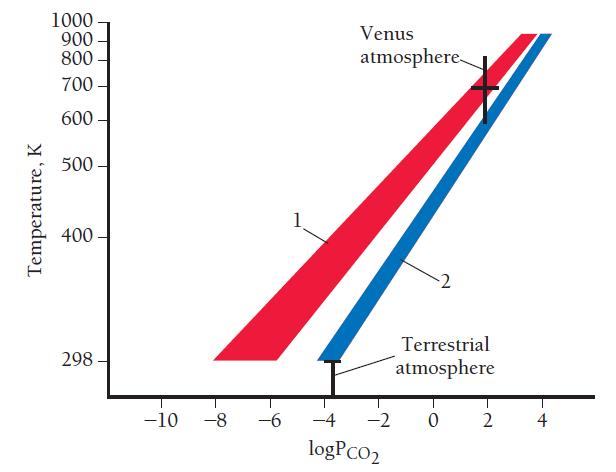
a. The partial pressure of carbon dioxide on the surface of Venus is 91 atm. What is the value of the equilibrium constant (Kp) if the Venusian carbon dioxide is in equilibrium according to system 1? According to system 2?
b. The approximate temperature on the surface of Venus is about 740 K. What is the approximate carbon dioxide concentration for system 1 at this temperature? For system 2?
c. Use the partial pressure of carbon dioxide on the surface of Venus given in part a to determine which of the two equilibrium systems is more likely to be responsible for the carbon dioxide on the surface of Venus.
d. From the carbon dioxide phase diagram, determine the minimum pressure required for supercritical carbon dioxide to form. If the partial pressure of carbon dioxide on the surface of Venus was higher in the distant past, could supercritical carbon dioxide have existed on the surface of Venus?
Step by Step Answer:





