The given reaction has a G rxn = 9.4 kJ at 25 C. Find G rx n
Question:
The given reaction has a ΔG°rxn = 9.4 kJ at 25 °C. Find ΔGrxn when PNO2 = 0.115 atm and PNO = 9.7 atm at 25 °C.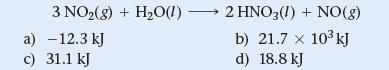
Transcribed Image Text:
3 NO₂(g) + H₂O(1) a) -12.3 kJ c) 31.1 kJ 2 HNO3(1) + NO(g) b) 21.7 x 10³ kJ d) 18.8 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
c...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Carol Harris, Ph.D, CPA, is a single taxpayer and she lives at 674 Yankee Street, Durham, NC 27409. Her Social Security number is 793-52-4335. Carol is an Associate Professor of Accounting at a local...
-
A researcher wanted to find out if there was difference between older movie goers and younger movie goers with respect to their estimates of a successful actors income. The researcher first...
-
Two tiny particles having charges of 5.76 C and -7 C are placed along the y-axis. The 5.76 C particle is at y = 0 cm, and the other particle is at y = 48.74 cm. Where must a third charged particle be...
-
As the CEO of your cookie company, you are interested in how public companies with a segment that includes cookies report their operating results. Because public companies are required to report on...
-
Show-Me-the-Money, Inc. is a medium-sized bank. The banks stock is owned primarily by residents in the city where the bank operates. During the last decade, the bank lent money for numerous real...
-
Write a code to test a Gaussian pseudorandom number generator. If you do not have a canned generator available, write a generator based on the Box-Muller algorithm in Appendix I. Apply the following...
-
Ocean Atlantic Co. is a merchandising business. The account balances for Ocean Atlantic Co. as of July 1, 2012 (unless otherwise indicated), are as follows: During July, the last month of the fiscal...
-
Following are the results from two different simple regression analyses, estimating the costs of the purchasing department using number of purchase orders and number of vendors as potential cost...
-
Based on its fundamental definition, explain why entropy is a measure of energy dispersion.
-
Find G rxn for the reaction 2 A + B 2 C from the given data. a) -401 kJ A B C2B C b) 509 kJ A AGxn 128 kJ AGxn = 455 kJ AGxn = -182 kJ c) 401 kJ = d) -509 kJ
-
Your client has systematically invested $1000 at the end of each half-year for the past 17 years. The invested funds have earned 6.4% compounded semiannually. What is the value of your clients...
-
Elucidate the correlation between an elevated diffusion coefficient and heightened gas solubility with regard to the consequent augmentation in diffusion rate, delineating the intricate mechanisms...
-
Kansas Corporation is reviewing an investment proposal that has an initial cost of $72,000. An estimate of the investment's end-of-year book value, the yearly after-tax net cash inflows, and the...
-
the response of the system depends on the poles of the transfer function. Create a block diagram in Simulink and use MATLAB to plot the response of the system to a pulse function with height of 0.03...
-
Calculate Sophie's taxable income in light of the following: Assessable income = $70,000 (source is salary and dividends); Donations to registered charities = $1,000; Donation to GoFund Me = $3,000;...
-
(1 point) Irene plans to retire on December 31st, 2019. She has been preparing to retire by making annual deposits, starting on December 31st, 1979, of $2400 into an account that pays an effective...
-
Discuss what have you learned as a result of completing this project and what would you do differently if you had to do it over? What advice would you give to other students who are embarking on this...
-
An auto-parts manufacturer is considering establishing an engineering computing center. This center will be equipped with three engineering workstations each of which would cost $25,000 and have a...
-
Figure P12.15 shows a snapshot of a wave on a string. This wave has a frequency of 200 Hz. Approximately how long does it take the wave crest located at point A in this snapshot to move to point B?...
-
When a sound wave passes from air into a solid object, which of the following wave properties stays the same and which change? Explain. (a) The amplitude (b) The frequency (c) The wave speed (d) The...
-
A woman wakes up just after 2 AM. Wondering what woke her, she goes to the window and looks around. Noting nothing out of the ordinary, she sits back down on her bed and all of a sudden a loud...
-
(1) provide an example of each lessonfrom the movie and (2) describe briefly how it illustrates/supports the lesson. Don't worry if you can't remember the characters' names. When a person's actions...
-
Transferable leadership skills The aim of this part of the assessment is for you to reflect on transferable leadership and professional skills that you may already have and/or need to develop. These...
-
A startup has 1,000 shares outstanding. A VC offers to invest $1 million at a post-money valuation of $5 million. How many shares will the VC get

Study smarter with the SolutionInn App


