The kinetics of this reaction were studied as a function of temperature. a. Determine the activation energy
Question:
The kinetics of this reaction were studied as a function of temperature.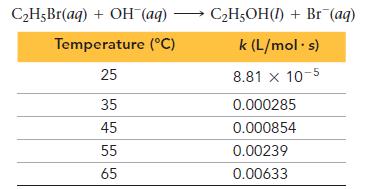
a. Determine the activation energy and frequency factor for the reaction.
b. Determine the rate constant at 15 °C.
c. If a reaction mixture is 0.155 M in C2H5Br and 0.250 M in OH-, what is the initial rate of the reaction at 75 °C?
Transcribed Image Text:
C₂H5Br(aq) + OH-(aq) Temperature (°C) 25 35 45 55 65 C2₂H5OH(1) + Br (aq) k (L/mol s) 8.81 x 10-5 0.000285 0.000854 0.00239 0.00633
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a Ea 895 kJm...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A second-order reaction has a rate constant of 8.7 104/(Ms) at 30oC. At 40oC, the rate constant is 1.8 103/(Ms). What are the activation energy and frequency factor for this reaction? Predict the...
-
For the reaction I (aq) + OCl (aq) OI (aq) + Cl (aq) occurring in aqueous solution, the following mechanism has been proposed: a. Derive the rate law expression for this reaction based on this...
-
The bond strength when mounting an integrated circuit on a metalized glass substrate was studied as a function of factor A = adhesive type, factor B = curve time, and factor C = conductor material...
-
Determine whether the given set of matrices under the specified operation, matrix addition or multiplication, is a group. Recall that a diagonal matrix is a square matrix whose only nonzero entries...
-
Refer to Exercise 5-6. Required: 1. Prepare journal entries for the August transactions. 2. Calculate the ending balances of each of the inventory accounts as of August 31.
-
A capacitor C, which may be considered to be loss-free, is connected with an inductor to form a closed loop. The inductor behaves as a pure inductance L in series with a resistor R. If, when the...
-
Refer to the information in Exercise 16-12. Prepare a process cost summary using the FIFO method. (Round cost per equivalent unit calculations to two decimal places.) Data From Exercise 16-12 The...
-
Evaluating a cost center including flexible budgeting concepts Smiley Medical Equipment Company makes a blood pressure measuring kit. Elbert Jackson is the production manager. The production...
-
ohn is preparing to play football at State University, which has a nationally ranked football program. He has been offered a generous scholarship, including 5 years of free tuition, free books, and...
-
The evaporation of a 120-nm film of n-pentane from a single crystal of aluminum oxide is zero order with a rate constant of 1.92 * 10 13 molecules/cm 2 s at 120 K. a. If the initial surface coverage...
-
The desorption (leaving of the surface) of a single molecular layer of n-butane from a single crystal of aluminum oxide is found to be first order with a rate constant of 0.128/s at 150 K. a. What is...
-
Elaine was working at a local mobile telecommunications companys retail outlet. Her main role was to help out potential customers with questions related to products and plans or existing customers...
-
What do the profit centers in responsibility reports mean?
-
Calculate the gross profit and operating income for June using absorption costing. Burlington Company reports the following information for June: Sales Revenue Variable Cost of Goods Sold Fixed Cost...
-
Give three benefits of a standard cost system.
-
What is the formula for calculating the predetermined overhead allocation rate?
-
How does indirect labor differ from indirect materials?
-
Use the following information to determine this companys cash flows from financing activities. a. Net income was $ 35,000. b. Issued common stock for $ 64,000 cash. c. Paid cash dividend of $ 14,600....
-
-4 1 9. Let A = Find A-1, (A") and verify that (A")= (A-1)".
-
When a beam of white light passes perpendicularly through a flat pane of glass, it is not dispersed into a spectrum. Why not?
-
Describe the image the lens of the eye forms on the retina.
-
The candle of Exercise 53 is 30 cm from the lens. Answer the same questions for this situation.
-
Over the period 19911993, IBM (US) suffered a net loss of almost $16 billion (half the total GDP of the Republic of Ireland at that time) see Figure 1.5 . During this period, the company had many of...
-
Having made their fortune playing online poker, Barryand Bob skip town to go to the island of Kokomo to bask in the sun and drink margaritas (with salt), taking with them all of their scholarly...
-
Lakeland Manufacturing Company has a patent on a high-pressure hydraulic valve. The valve is a major improvement in high-pressure hydraulic systems, and Lakeland has enjoyed a monopoly position in...

Study smarter with the SolutionInn App


