The reaction A(g) B(g) has an equilibrium constant that is less than one. What can you
Question:
The reaction A(g) ⇌ B(g) has an equilibrium constant that is less than one. What can you conclude about ΔG°rxn for the reaction?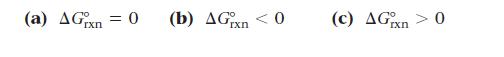
Transcribed Image Text:
(a) ΔGxn = 0 (b) ΔGxn < 0 (c) ΔGxn > 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
c Since the equilibrium consta...View the full answer

Answered By

Amar Kumar Behera
I am an expert in science and technology. I provide dedicated guidance and help in understanding key concepts in various fields such as mechanical engineering, industrial engineering, electronics, computer science, physics and maths. I will help you clarify your doubts and explain ideas and concepts that are otherwise difficult to follow. I also provide proof reading services. I hold a number of degrees in engineering from top 10 universities of the US and Europe.
My experience spans 20 years in academia and industry. I have worked for top blue chip companies.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The effectiveness of mixing within a batch reactor or other stirred tank is determined mainly by the stirring power. The power is the rate at which work is done on the liquid by the moving impeller....
-
The reaction A( g) B(g) has an equilibrium constant of 5.8 and under certain conditions has Q = 336. What can you conclude about the sign of G rxn and G rxn for this reaction under these conditions?
-
Consider a repeated version of the game in exercise 24.5. In this version, we do not give all the proposal power to one person but rather imagine that the players are bargaining by making different...
-
To the right is the graph of the position of the object versus time: Which of graphs below correctly shows the object's velocity versus time? velocity (m/s) velocity (m/s) 1.5 1 0.5 -0.5 -1 -1.5 15...
-
Motorola, Inc., originated a $5 billion project, called Iridium, that launched 66 low-earth-orbit satellites for global communication using pagers and mobile phones. After its operations began, the...
-
Three companies have the following financial results: What can you conclude about the financial results and the operating strategy of eachcompany? Company A Company B Profit margin Asset turnover...
-
Mr. Jenkins, this is typical question: Do you feel that I have treated you fairly in this interview?
-
Victory Company uses weighted- average process costing to account for its production costs. Direct labor is added evenly throughout the process. Direct materials are added at the beginning of the...
-
Company EJ plans to build a new plant to manufacture bicycles. EJ sells its bicycles in the world market for $400 per bike. It could locate the plant in Province P, which levies a 20 percent tax on...
-
Use standard free energies of formation to calculate G rxn for the balanced chemical equation: Mg(s) + NO(g) Substance NO(g) MgO(s) a) 673.0 kJ b) -673.0 kJ c) -465.6 kJ d) 465.6 kJ MgO(s) + N(g)...
-
What is the precise definition of entropy? What is the significance of entropy being a state function?
-
Mr Michael Quint has recently taken over the family owned, independent producer of quality wines in Germanys picturesque Mosel River Valley. With the enthusiastic support of his wife and the...
-
How does the negotiation process influence organizational behavior and management in criminal justice organizations? Provide an example to substantiate your rationale.
-
The Woodson Theater sells season theater tickets for $200 each. Each ticket entitles the customer to attend 5 plays. The first play will be in December. On December 1, 20X1, 400 season tickets were...
-
Which conflict management styles are effective in criminal justice organizations? Provide an example to substantiate your rationale.
-
This firm is at the bottom of the barrel from customer perspective. Loser B) Nonentity C Professional D Leader
-
Give 6 risks of a Tesla expansion in the Philippines, and provide mitigation strategies for each risk.
-
Supply chain relationships have three dimensions: people, processes, and platforms. Which of these do you think is the most critical? In what way?
-
Data 9.2 on page 540 introduces the dataset Cereal, which includes information on the number of grams of fiber in a serving for 30 different breakfast cereals. The cereals come from three different...
-
In a sound wave in a solid, the atoms in the solid move parallel to the propagation direction. Does that means the atoms travel along with the wave? Explain why or why not.
-
Waves on a violin string travel at 900 m/s. If the string has a tension of 50 N and is 0.33 m long, what is its mass?
-
For guitars and violins, some strings are simple strings, and others contain a winding around a string core. Which type of string, simple or wound, is used for the low-frequency notes? Explain why.
-
Win Win Limited is a robot manufacturer to be sold to another tech firm at the end of one year. Win Win expects that the net cash flow at the end of one year will be USD40 million, a record high of...
-
The project team is not functioning as a team". This was one of Jack's key findings in his assessment of why the project is running behind schedule and over budget. If you were in Jack's position....
-
Use the following corn futures quotes: Contract Month Mar Corn 5,000 bushels Open High Low 455.125 457.000 May Settle 451.750 452.000 -2.750 467.000 468.000 463.000 463.250 -2.750 Chg Open Int...

Study smarter with the SolutionInn App


