The reactions shown here can be combined to make the overall reaction C(s) + H 2 O(
Question:
The reactions shown here can be combined to make the overall reaction C(s) + H2O( g) → CO(g) + H2( g) by reversing some and/or dividing all the coefficients by a number. As a group, determine how the reactions need to be modified to sum to the overall process. Then have each group member determine the value of K for one of the reactions to be combined. Finally, combine all the values of K to determine the value of K for the overall reaction.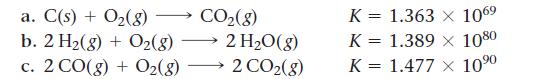
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





