The structure of caffeine, present in coffee and many soft drinks, is shown here. How many pi
Question:
The structure of caffeine, present in coffee and many soft drinks, is shown here. How many pi bonds are present in caffeine?
How many sigma bonds? Insert the lone pairs in the molecule.
What kinds of orbitals do the lone pairs occupy?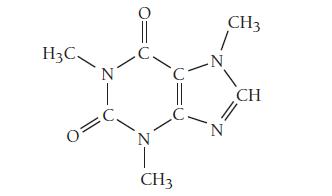
Transcribed Image Text:
H3C 0 N 0 'C CH3 -N N CH3 CH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
o bonds 25 bonds 4 lon...View the full answer

Answered By

Muhammad Mahtab
everyone looks that their work be perfect. I have more than a five year experience as a lecture in reputable institution, national and international. I provide perfect solution in marketing, case study, finance problems, blog writing, article writing, business plans, strategic management, human resource, operation management, power point presentation and lot of clients need. Here is right mentor who help clients in their multi-disciplinary needs.
5.00+
3+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Family Farms exchanged an old tractor for a newer model. The new tractor is similar in nature to the old one and therefore there is no economic substance to the transaction. Amounts Cost of old...
-
Glycine, is an amino acid used in the synthesis of proteins. Its molecular formula is C 2 H 5 NO 2 . Below is a partial arrangement of the atoms of glycine. N C C O a. Complete the Lewis structure of...
-
What is your qualitative assessment of Twitter's current and prospective business? What is your assessment of Twitter's value at the time of the acquisition? Use the comparable companies approach....
-
The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is formed when the conjugate base of diethyl malonate reacts with...
-
The management of Weimar Inc., a civil engineering design company, is considering an investment in a high-quality blueprint printer with the following cash flows: Required: 1. Determine the payback...
-
Calculating Annuity Present Value An investment offers $4,600 per year for 15 years, with the first payment occurring one year from now. If the required return is 8 percent, what is the value of the...
-
Petitioner Atlantic Marine Company was a contractor located in Virginia. It entered into a subcontract with J-Crew Management, Inc., a Texas corporation, that contained a forum-selection clause...
-
Listed below are the budgeted factory overhead costs for 2011 for Muncie Manufacturing, Inc., at a projected level of 2,000 units: Expenses: Indirect materials . . . . . . . . . . . . . . . . . . . ....
-
Suppose A utility function U(x) = {ax{ +(1-)x2}" with a > 0 and p>0 is defined over the set S = {(x) | xp+x2P2 M} where P, P2 are prices. Prove that if P1, P2 and M are positive, there exists at...
-
The structure of acetylsalicylic acid (aspirin) is shown here. How many pi bonds are present in acetylsalicylic acid? How many sigma bonds? What parts of the molecule are free to rotate? What parts...
-
The genetic code is based on four different bases with the structures shown here. Assign a geometry and hybridization to each interior atom in these four bases. a. Cytosine b. Adenine c. Thymine d....
-
What are the four types of consumer products? How do marketing considerations vary by consumer product type?
-
Define and distinguish between future value and present value.
-
Without authority to do so, Allen signed Bakers name on a note, promising to pay Cohen $5,000 in 30 days. The note was negotiated to Davidson who was a holder in due course. Will Davidson be...
-
What are the influences on the supply of U.S. dollars in the foreign exchange market?
-
Colombia is the worlds biggest producer of roses. The global demand for roses increases and at the same time Columbias central bank increases the interest rate. In the foreign exchange market for...
-
How is the supply of money determined and how does it depend on the Feds monetary policy strategy?
-
What is the most ethical way to do business internationally?
-
In Problems 718, write the augmented matrix of the given system of equations. f0.01x0.03y = 0.06 [0.13x + 0.10y = 0.20
-
The radius of the pedestal is defined by r = (0.5e 0.08y2 )m, where y is in meters. If the material has a density of 2.5 Mg/m 3 , determine the average normal stress at the support. Fr= 0.5e 0.08y?...
-
Determine the greatest constant angular velocity Ï of the flywheel so that the average normal stress in its rim does not exceed Ï = 15 MPa. Assume the rim is a thin ring having a thickness...
-
If the allowable tensile stress for wires AB and AC is Ï allow = 200 MPa, determine the required diameter of each wire if the applied load is P = 6 kN. 45
-
Write the feature of Super's Career Development Assessment and Counseling (C-DAC) system?
-
Describe one type of role-play technique you could use in a specific group setting. Explain who would benefit from this technique and why. Toseland, R. W., & Rivas, R. F. (2017). An introduction to...
-
Suppose that a manager is following a base stock policy where the optimal inventory position is 10. Assume the component lead time is 2 days. At the end of day 1, there is no ordered units yet to be...

Study smarter with the SolutionInn App


