The titration of 10.00 mL of HCl solution of unknown concentration requires 12.54 mL of a 0.100
Question:
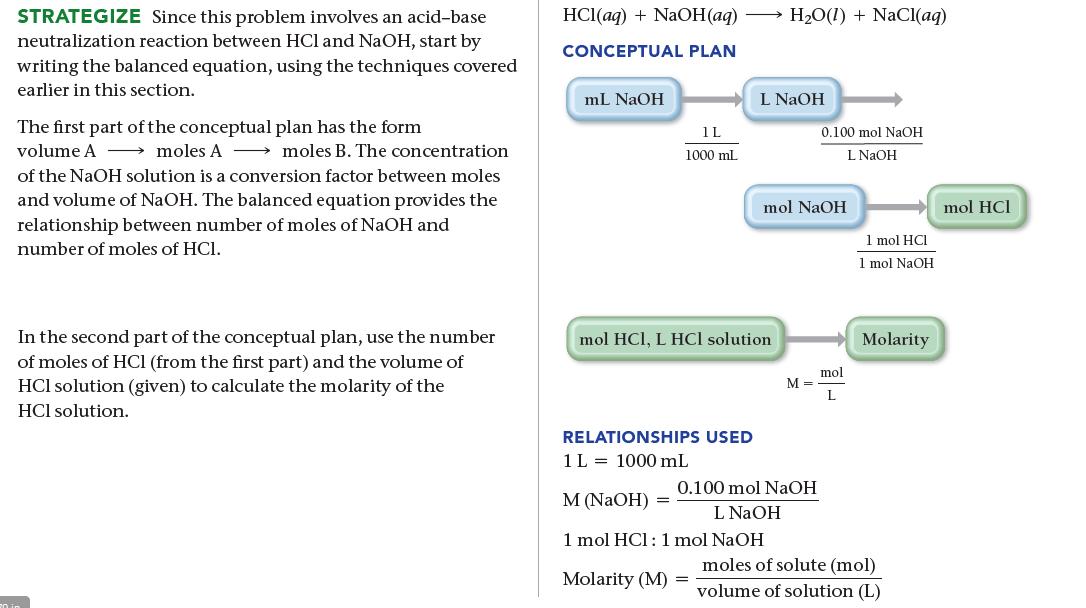
The titration of 10.00 mL of HCl solution of unknown concentration requires 12.54 mL of a 0.100 M NaOH solution to reach the equivalence point. What is the concentration of the unknown HCl solution in M?
Transcribed Image Text:
SORT You are given the volume and concentration of NaOH solution required to titrate a given volume of HCl solution. You are asked to find the concentration of the HCl solution. GIVEN: 12.54 mL of NaOH solution, 0.100 M NaOH solution, 10.00 mL of HCl solution FIND: concentration of HCl solution
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
1254 ml NaOH X Molarity 1 L 1000 mL 1 mol HCI 1 mol NaOH 0100 molNaOH LNaOH 125 ...View the full answer

Answered By

Hamza Amjad
Currently I am student in master degree program.from last two year I am tutring in Academy and I tought many O/A level student in home tution.
4.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
The flask contains 25 mL of an unknown diprotic acid aqueous solution that reacts in a 1:2 stochiometric ratio with NaOH. Titrate the solution with NaOH to determine the concentration of the acid....
-
A procedure15 for determining halogens in organic compounds uses an argentometric titration. To 50 mL of anhydrous ether is added a carefully weighed sample (10 - 100 mg) of unknown, plus 2 mL of...
-
dentify a true statement about penetration pricing. It emphasizes a company's current performance but can sacrifice long-term performance. It requires a company to price goods to cover variable costs...
-
Are the inputs and outputs of a production process likely to be different for a home builder than for a cement company? How?
-
The Gourmand Cooking School runs short cooking courses at its small campus. Management has identified two cost drivers that it uses in its budgeting and performance reportsthe number of courses and...
-
Mrs. Palsgraf was waiting for a train on a platform of a railroad. When a different train came into the station, two men ran to get on that train before it left the station. While one of the men...
-
How important are excellent leaders to organizations? A company has to cultivate leaders who have the skills and abilities to help it survive and thrive. 3M has its own farm system, except its farm...
-
if assets are 1 1 5 , 0 0 0 , owner investments are 2 9 , 0 0 0 , loss of 2 5 , 5 0 0 and owner withdrawals are 6 , 9 0 0 . what are the liabilities
-
Which statement best describes the difference between the charge of a polyatomic ion and the oxidation states of its constituent atoms? (For example, the charge of NO 3 - is 1, and the oxidation...
-
Write a molecular equation, ionic equation, and net ionic equation for the reaction between aqueous acetic acid (HC 2 H 3 O 2 ) and aqueous potassium hydroxide (KOH).
-
From your conclusions in Problem, decide which of the direction fields in Fig. 2.2.4 represent linear homogeneous equations. Explain your reasons in each case (A) (B) (D) (E) )
-
Problems in learning within organizations that contribute to the bullwhip effect are referred to as incentive obstacles. information processing obstacles. pricing obstacles. behavioral obstacles.
-
Retailing in the United States is largely push/pull. consolidated. profitable. centralized.
-
Promotion decisions are often made by retailers without accounting for the impact on the rest of the supply chain. the retailers profit margin. stock levels. seasonal variation.
-
For any supply chain, the source of revenue is generated by efficient operations. information flows. the customer. product flows.
-
Weekly demand for cell phones at a Best Buy store is normally distributed, with a mean of 300 and a standard deviation of 200. The supplier takes two weeks to supply a Best Buy order. Best Buy is...
-
On December 31, 2010, the Stevens Company bookkeeper prepared the following erroneously classified balance sheet: Required You determine that the account balances listed on the balance sheet are...
-
The registrar of a college with a population of N = 4,000 full-time students is asked by the president to conduct a survey to measure satisfaction with the quality of life on campus. The following...
-
Imagine a sound wave with a frequency of 1.10 kHz propagating with a speed of 330 m/s. Determine the phase difference in radians between any two points on the wave separated by 10.0 cm.
-
Given the traveling wave (x, t) = 5.0 exp (-x 2 - bt 2 - 2b xt), determine its direction of propagation. Calculate a few values of and make a sketch of the wave at t = 0, taking = 25 m -2 and b =...
-
Determine which of the following describe traveling waves: (a) (b) (c) (d) Where appropriate, draw the profile and find the speed and direction of motion. (y, t) = ea*y+br2abty) | V(z, t) = A sin (az...
-
How can Total Quality Management be applied in the service industry, and what unique considerations should service-based organizations take into account during the implementation process?
-
Describe the management of EHRs including eliminating duplicate records, purging records, and backing up the EHR.
-
Settings for Bills and Expenses include: Multiple Choice Show items table on expense and purchase forms. Track expenses and items by customer. Track billable expenses and items as income. All of the...

Study smarter with the SolutionInn App


