The vapor pressure of a 1 M ionic solution is different from the vapor pressure of a
Question:
The vapor pressure of a 1 M ionic solution is different from the vapor pressure of a 1 M nonelectrolyte solution. In both cases, the solute is nonvolatile. Which set of diagrams best represents the differences between the two solutions and their vapors?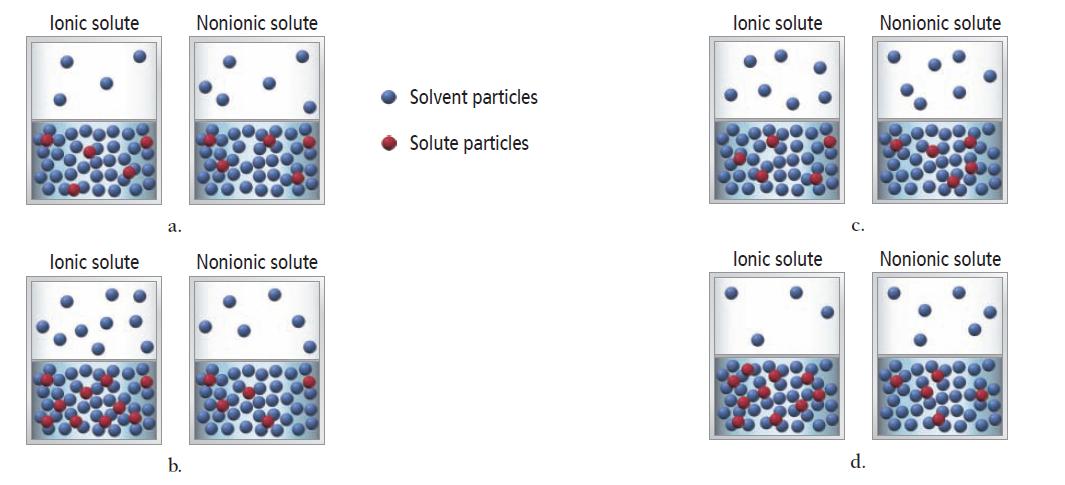
Transcribed Image Text:
lonic solute lonic solute a. b. Nonionic solute Nonionic solute Solvent particles Solute particles lonic solute lonic solute C. d. Nonionic solute Nonionic solute
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The correct set of diagrams that best represents the differences between the 1 M ionic solution and the 1 M nonelectrolyte solution and their vapors i...View the full answer

Answered By

CHARLES AMBILA
I am an experienced tutor with more than 7 years of experience. I have helped thousands of students pursue their academic goals. My primary objective as a tutor is to ensure that students have easy time handling their academic tasks.
5.00+
109+ Reviews
323+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The vapor pressure of various substances can be determined using effusion. In this process, the material of interest is placed in an oven (referred to as a Knudsen cell) and the mass of material lost...
-
What is the effect of a nonvolatile solute on the vapor pressure of a liquid? Why is the vapor pressure of a solution different from the vapor pressure of the pure liquid solvent?
-
What is the vapor pressure of a solution in which the mole fraction of the solute is 0.200 and the vapor pressure of the pure solvent is 100.0 torr? (Assume a single nonvolatile, nonelectrolyte...
-
Integrate using an appropriate formula For each problem, state the formula number, u and du, identify any constants (if appropriate), and show any constant "adjustments" / "Multiply by 1" if needed....
-
The LeVitre and Swezey Credit Union maintains separate bank accounts for each of its 20,000 customers. Three major files are the customer master file, the transaction file of deposits and withdrawal...
-
A straight rod of negligible mass is mounted on a frictionless pivot as in Figure. Masses m 1 and m 2 are suspended at distances l 1 and l 2 .? (a) Write an expression for the gravitational potential...
-
In 1988, the Upper Deck Company was a company with an idea for a better baseball card: one that had a hologram on it. By the 1990s, the firm was a major corporation worth at least a quarter of a...
-
Metro Shuttle Company is considering investing in two new vans that are expected to generate combined cash inflows of $28,000 per year. The vans combined purchase price is $91,000. The expected life...
-
Explain how specific institutions of capitalism can harness self interest to benefit the common good and improve economic outcomes in society at large. Explain what a social dilemma is and how it...
-
If each substance listed here costs the same amount per kilogram, which would be most cost-effective as a way to lower the freezing point of water? (Assume complete dissociation for all ionic...
-
A power plant built on a river uses river water as a coolant. The water is warmed as it is used in heat exchangers within the plant. Should the warm water be immediately cycled back into the river?...
-
Research Equipment Inc. (REC) is a small manufacturer of precision tools used to construct research equipment for engineering departments at colleges and universities. It sells its two main products,...
-
Reconsider question 50. This time, assume that the standard deviation is not known and that .5 oz is the sample standard deviation. Again construct a 99 % confidence interval for the mean amount of...
-
A marketing consulting company wants to estimate the percentage of students holding credit cards by sending questionnaires to students. The sponsor of this research wants to establish a 95 %...
-
A random sample of 450 people who took Dollar Daves CPA review course reveals that 310 of them passed the CPA exam on the first try. Construct a 90 % confidence interval for the proportion of people...
-
Use the information given in question 62 to construct a 90 % confidence interval for the proportion of rolls that will come up 6. Question 62 Suppose a golfer on the University of Houston golf team...
-
Go to the St. Louis Federal Reserve FRED database, and find data on exports (BOPXGS) and imports (BOPMGS). a) Calculate net exports for the most current period available. Is the United States...
-
What would cause (a) a steep ADI curve; (b) a gently sloping ADI curve? Compare the short-run and longrun effects of (i) a temporary adverse supply shock and (ii) a permanent supply reduction under...
-
The Zwatch Company manufactures trendy, high-quality moderately priced watches. As Zwatch's senior financial analyst, you are asked to recommend a method of inventory costing. The CFO will use your...
-
Starting with Eq. (3.32), prove that the energy densities of the electric and magnet fields are equal (u E = u B ) for an electromagnetic wave. (3.32) UB 2o
-
Prove that the irradiance of a harmonic EM wave in vacuum is given by and then determine the average rate at which energy is transported per unit area by a plane wave having an amplitude of 15.0 V/m....
-
A nearly cylindrical laserbeam impinges normally on a perfectly absorbing surface. The irradiance of the beam (assuming it to be uniform over its cross section) is 40 W/cm 2 . If the diameter of the...
-
In late adulthood, there are changes that occur physically and cognitively which are at times difficult for the individual to embrace. Please share at least2physical (one must relate to sex or...
-
Managing Human Resources 1. What have you learned ? 2. Discuss why managers have difficulties in managing human resources? 3. How can we achieve employee commitment?
-
What are the advantages and disadvantages of a dress code policy in the workplace?

Study smarter with the SolutionInn App


