These images represent the first-order reaction A B initially and at some later time. The rate
Question:
These images represent the first-order reaction A → B initially and at some later time. The rate law for the reaction is Rate = 0.010 s-1 [A]. How much time has passed between the two images?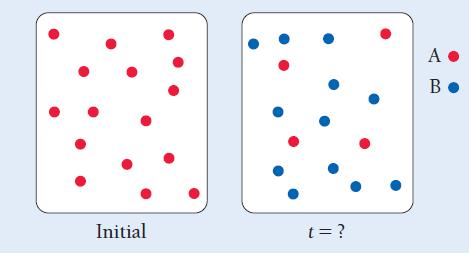
a) 69 s
b) 139 s
c) 60 s
d) 12.5 s
Transcribed Image Text:
Initial t = ? A. B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
b...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Required: a. Assuming that the expectations hypothesis is valid, compute the expected price of the four-year zero coupon bond shown below at the end of (i) the first year; (ii) the second year; (iii)...
-
If X has the distribution function Find (a) P(X 3); (b) P(X = 3); (c) P(X < 3); (d) P(X 1); (e) P(- 0.4 < X < 4); (f) P(X = 5). F(x) = for 1 x <3 for 3 Sr5 1 for 5
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Why is it so difficult to assess whether a firm is properly valued?
-
After graduating, you might decide to start a small business. As discussed in this chapter, owners of any business need to know how to calculate the cost of their products. In fact, many small...
-
What dimensions are used to segment business markets?
-
The trial balance for Gold Rush Exploration Company, Inc. does not balance. The following errors were detected: a. The cash, balance is overstated by \($2,000\). b. Rent expense of \($390\) was...
-
Kirsten Neal is interested in purchasing a new house given that mortgage rates are at a historical low. Her bank has specific rules regarding an applicants ability to meet the contractual payments...
-
Duncan and Feyd start investing at the same time. Duncan makes payments of $40 at the end of each week into an investment that earns 5.25%, compounded weekly. Feyd makes a single investment into an...
-
Explain the meaning of each term within the Arrhenius equation: activation energy, frequency factor, and exponential factor. Use these terms and the Arrhenius equation to explain why small changes in...
-
How do reaction rates typically depend on temperature? What part of the rate law is temperature dependent?
-
How did the Sarbanes-Oxley Act attempt to reduce fraudulent reporting by addressing opportunity, incentives, and character?
-
Data for Hermann Corporation are shown below: Selling price Variable expenses Contribution margin Percent of Per Unit $ 100 61 Sales 100% 61 $ 39 39% Fixed expenses are $80,000 per month and the...
-
How did the great compromise impact the south political power when combined with the 3 / 5 compromise?
-
2 . In the month of Feb, the company had a net income of Php 5 2 5 , 1 0 0 from the sale of Php 1 , 3 0 0 , 0 0 0 . The total conversion cost is Php 4 7 1 , 9 0 0 and the total indirect cost is Php 3...
-
What strategies can be employed to maximise the impact of a limited budget in the tourism industry?
-
How is share price of a stock - exchange - listed corporation estimated using the earnings multiple?
-
What is the present value of $ 10,000 five years from today if interest is? A. 10 percent compounded annually? B. 10 percent compounded semiannually? C. 10 percent compounded quarterly? D. Why do the...
-
Borrowing costs should be recognised as an expense and charged to the profit and loss account of the period in which they are incurred : A. If the borrowing costs relate to qualifying asset B. If the...
-
Add the two waves of Problem 7.7 directly to find Eq. (7.17). Data from Problem 7.7 Using Eqs. (7.9), (7.10), and (7.11), show that the resultant of the two waves and is k r x E = 2E01 cos (7.17) sin...
-
Use the complex representation to find the resultant E = E 1 + E 2 , where E 1 = E 0 cos (k x + t) and E 2 = -E 0 cos (k x - t) Describe the composite wave.
-
Consider the functions E 1 = 3 cos Ït and E 2 = 4 sin Ït. First prove that E 2 = 4 cos (Ït - Ï/2). Then, using phasors and referring to Fig. P.7.10, show that E 3 = E 1 + E 2 = 5...
-
Describe the client organisation considerations when designing a wireless network.?
-
2. A program is designed to compute a customer's car rental charges. Every car is charged at RM25 per day with addition to a charge of RM 0.40 for every kilometer used. Finally, the final rental...
-
Write the loop invariant for the following code: item = -INF (minus infinite) for (i=0 to n-1) if (A[i] item) item =A[i]

Study smarter with the SolutionInn App


