Two 25.0-mL samples of unknown monoprotic weak acids, A and B, are titrated with 0.100 M NaOH
Question:
Two 25.0-mL samples of unknown monoprotic weak acids, A and B, are titrated with 0.100 M NaOH solutions.
The titration curve for each acid is shown below. Which of the two weak acid solutions is more concentrated? Which of the two weak acids has the larger Ka?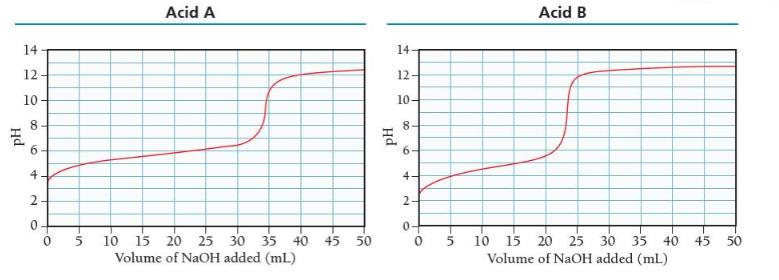
(a) Acid B is more concentrated and has the larger Ka.
(b) Acid A is more concentrated and has the larger Ka.
(c) Acid A is more concentrated and Acid B has the larger Ka.
(d) Acid B is more concentrated and Acid A has the larger Ka.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





