Upon combustion, a compound containing only carbon and hydrogen produces 1.83 g CO 2 and 0.901 g
Question:
Upon combustion, a compound containing only carbon and hydrogen produces 1.83 g CO2 and 0.901 g H2O. Find the empirical formula of the compound.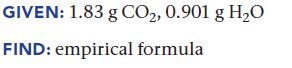
Transcribed Image Text:
GIVEN: 1.83 g CO₂, 0.901 g H₂O FIND: empirical formula
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
183 g CO X 1 mol CO 4401 g CO 00416 mol CO 0901 g HO X 1 mol HO 1802 ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A compound containing only carbon and hydrogen produces 113.49 g CO2 and 54.36 g H2O when combusted. What is the empirical formula of this compound? If it has a molar mass of 86.20 g/mol, what is its...
-
Write a program "three.c" which contains a function (named "three") that takes two arguments: a pointer to an int and a pointer to a char. The function should return a float. This function should...
-
A compound containing only carbon and hydrogen is 85.63% C by mass. Reaction of this compound with H2O produces a secondary alcohol as the major product and a primary alcohol as the minor product. If...
-
Burger Prince buys top-grade ground beef for $1.00 per pound. A large sign over the entrance guarantees that the meat is fresh daily. Any leftover meat is sold to the local high school cafeteria for...
-
Suppose you have a project with a payback period exactly equal to the life of the project. What do you know about the IRR of the project? Suppose that the payback period is never. What do you know...
-
Consider the conducting rod of Example 1.3 under steady-state conditions. As suggested in Comment 3, the temperature of the rod may be controlled by varying the speed of air flow over the rod, which,...
-
Parents of minors took Apple to court in 2012 for supplying game applications, on iPhones, that were free but through which users could purchase in-game currencies. Apparently, parents would log on...
-
At week 24 of a project to shoot a television commercial, what should the expenditures be? If the earned value is right on schedule but the actual expenses are $9,000, what are the cost and schedule...
-
How to convert this Entity-Relationship data model into a relational database model? Contains Inventory Inventory ID Customer Cust_ID Amount Availible Cust LName Must Have Coffee_Type Cust Fname...
-
On January 1, 2014, Merchant Co. sold a tractor to Swanson Inc. and simultaneously leased it back for five years. The tractors fair value is $250,000, but its carrying value on Merchants books prior...
-
What is the difference between an alkane, an alkene, and an alkyne?
-
Which elements are normally present in organic compounds?
-
Fillon operates manufacturing facilities in States A and B. Fillon has nexus with both states; apportionment factors are .70 for A and .30 for B. Taxable income for the year totaled $150,000, with a...
-
Financial aid debt and depression Write the problem statement. Describe the two recommendations you proposed in this week's Generating Potential Solutions discussion forum. Select the best potential...
-
On January 31, 2022, Lenny sold an apartment building for $217,500. He had purchased the building on February 1, 2021, for $192,500. The depreciation deducted as of January 31, 2022, was $7,000. What...
-
Most buyers in the futures market take delivery of the underlying asset. Question 15 options: True False
-
A person draws a single card from a 52-card deck. Consider the following two events: Drawing a black card (club or spade) Mutually exclusive? Prove mathematically. Independent? Prove mathematically....
-
1 What influences strategy development in Google?
-
The management of Dorsch Aluminum Co. is considering whether to process aluminum ingot further into rolled aluminum. Rolled aluminum can be sold for $4,100 per ton, and ingot can be sold without...
-
Shreemaya Hotel in !adore was facing a problem of low demand for its rooms due to off season. The Managing Director (MD) of the hotel, Mrs. Sakina was very worried. She called upon the Marketing...
-
Why is it reasonable to write dH C P dT + VdP for a liquid or solid sample?
-
For each of the following pairs of compounds, identify the higher boiling compound and justify your choice: a. b.
-
Refer to Figure 1.10 and explain why (U/V) T is generally small for a real gas. Figure 1.10 Ideal gas Real gas Tranlaition rv-0 (4)A
-
Suppose the Texas legislature wants to deal with concerns about college affordability. They pass a law that gives every resident in Texas two free years of college education at public colleges and...
-
The unemployment rate among workers under 25 in a populous state went from 8.9% to 6.7% in one year. Assume an average of 1 comma 340 comma 500 workers and estimate the decrease in the number...
-
Suppose you have 135 households in your neighborhood, can you use "=RAND()" function in Excel to randomly select 20 of them to send a survey? a) Write down the codes you would use. b) Show your work...

Study smarter with the SolutionInn App


