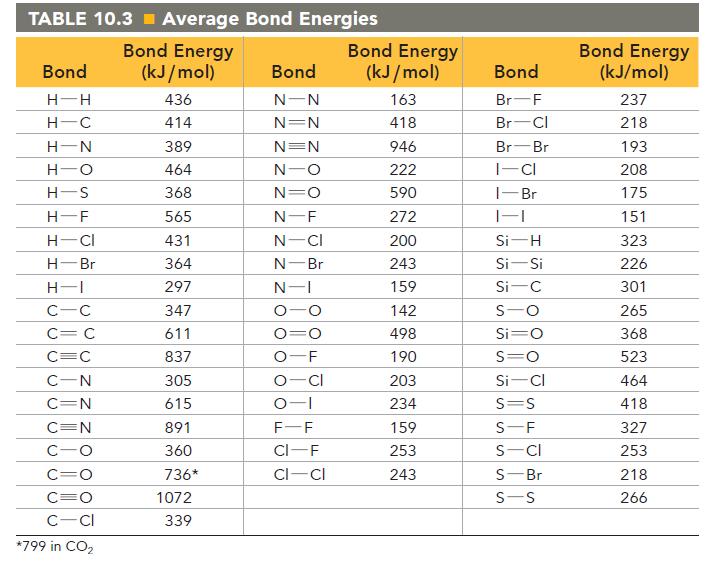
Use bond energies from Table 10.3 to determine H rxn for the reaction between ethanol and hydrogen
Question:
Use bond energies from Table 10.3 to determine ΔHrxn for the reaction between ethanol and hydrogen chloride.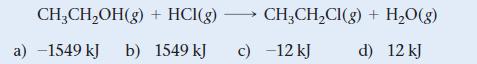
Transcribed Image Text:
CH₂CH₂OH(g) + HCI(g) →→→→→ CH₂CH₂Cl(g) + H₂O(g) b) 1549 kJ c) -12 kJ d) 12 kJ a) -1549 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
c...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Resonance energy is the difference in energy between a real moleculea resonance hybridand its most important contributing structure. To determine the resonance energy for benzene, we can determine an...
-
a. Write a balanced equation to show the reaction between ethanol and hydrogen bromide. b. What are the reagents and conditions used for this reaction? c. What do we call this type of reaction?
-
Hydrazine (N 2 H 4 ) is used as a fuel in liquid-fueled rockets. When hydrazine reacts with oxygen gas, nitrogen gas and water vapor are produced. Write a balanced equation and use bond energies from...
-
A 14-foot piece of string is cut into two pieces so that the longer piece is 2 feet longer than twice the shorter piece. Find the lengths of both pieces. What is the lenath of the shorter oiece?1...
-
Whats going on in that lab? asked Derek Warren, chief administrator for Cottonwood Hospital, as he studied the prior months reports. Every month the lab teeters between a profit and a loss. Are we...
-
A continuous-time signal x c (t) is bandlimited to 5 kHz; i.e., X c (j) = 0 for || 2 (5000). x c (t) is sampled with period T, producing the sequence x [n] = x c (nT). To examine the spectral...
-
Linda Budd went searching for a new friend and she found one for \($400.1\) A brand new puppy. She purchased the puppy from Bernadette Vicidomine, a person who regularly sells puppies. Budd took her...
-
Malone Company estimates that 360,000 direct labor hours will be worked during the coming year, 2010, in the Packaging Department. On this basis, the following budgeted manufacturing overhead cost...
-
Abbey Lee is driving north to a dance competition for 3 hours at 46 miles per hour. Meanwhile, leaving from the same place, Cathy is driving to a different dance competition 2 hours south at 43 miles...
-
In a covalent Lewis structure, what is the difference between lone pair and bonding pair electrons?
-
How does lattice energy relate to ionic radii? To ion charge?
-
Roy Rand executed and delivered the following note to Sue Sims: "Chicago, Illinois, June 1, 2017; I promise to pay to Sue Sims or bearer, on or before July 1, 2017, the sum of $7,000. This note is...
-
Discuss the view that the distinctive characteristics of business structures and institutions create the case for distinctively different accounting law and practice in Japan.
-
Compare the cost/performance ratio with and without this improvement. When processor designers consider a possible improvement to the processor datapath, the decision usually depends on the...
-
Determine the effect on short-run aggregate supply for each of the following events. Explain whether it represents a movement along the SRAS curve or a shi of the SRAS curve. a. A rise in the...
-
Discuss how the development of accounting regulation in China has reflected the growth of interaction with global economies.
-
If you or someone you know bought a new car recently, the odds are pretty good that it was manufactured by one of two Japanese companies, Toyota or Honda. Together, these companies account for about...
-
On January 1, 2010, Von Company entered into two noncancelable leases for new machines to be used in its manufacturing operations. The first lease does not contain a bargain purchase option. The...
-
Write a function that reads a Float24_t value: Float24_t float24_read(void) A legitimate float24 value string is of the form: "mantissabexponent" where the mantissa (m) and the exponent (e) may have...
-
A shear force of V = 18 kN is applied to the box girder. Determine the shear flow at point C. 10 mm 30 mm 10 mm 100 mm B. 150 mm 100 mm 10 mm 30 mm 10 mm 150 mm 10 mm 125 mm 10 mm
-
The aluminum strut is 10 mm thick and has the cross section shown. If it is subjected to a shear of V = 150 N, determine the shear flow at points A and B. 10 mm 40 mm 10 mm t+-40 mm- 30 mm 30 mm 10...
-
The aluminum strut is 10 mm thick and has the cross section shown. If it is subjected to a shear of V = 150 N, determine the maximum shear flow in the strut. 10 mm 40 mm 10 mm tr-40 mm- 30 mm 30 mm...
-
For this question, you should type which formula you should use (simple, compound, annuity, payout annuity, loan). You should also tell me which variable you will solving for, and you should type...
-
You want to be able to withdraw the specified amount periodically from a payout annuity with the given terms. Find how much the account needs to hold to make this possible. Round your answer to the...
-
Following are the individual financial statements for Gibson and Davis for the year ending December 31, 2024: Account Sales Cost of goods sold Operating expenses Dividend income Net income Retained...

Study smarter with the SolutionInn App


