Use Coulombs law to calculate the ionization energy in kJ/mol of an atom composed of a proton
Question:
Use Coulomb’s law to calculate the ionization energy in kJ/mol of an atom composed of a proton and an electron separated by 100.00 pm. What wavelength of light has sufficient energy to ionize the atom?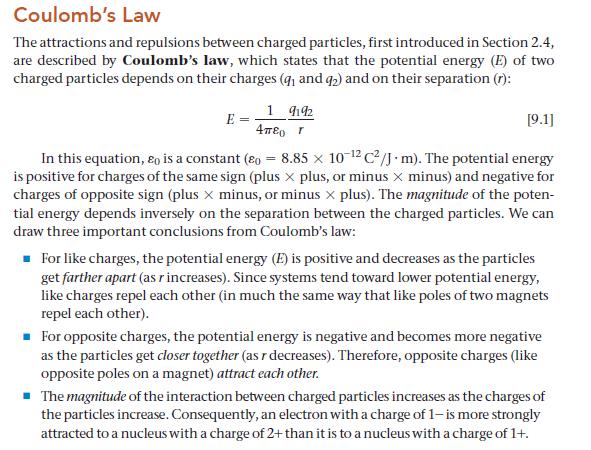
Transcribed Image Text:
Coulomb's Law The attractions and repulsions between charged particles, first introduced in Section 2.4, are described by Coulomb's law, which states that the potential energy (E) of two charged particles depends on their charges (9₁ and 92) and on their separation (r): 19192 E [9.1] In this equation, Eo is a constant (o= 8.85 x 10-12 C²/J.m). The potential energy is positive for charges of the same sign (plus x plus, or minus x minus) and negative for charges of opposite sign (plus x minus, or minus x plus). The magnitude of the poten- tial energy depends inversely on the separation between the charged particles. We can draw three important conclusions from Coulomb's law: Απερ r ■ For like charges, the potential energy (E) is positive and decreases as the particles get farther apart (as r increases). Since systems tend toward lower potential energy, like charges repel each other (in much the same way that like poles of two magnets repel each other). ■ For opposite charges, the potential energy is negative and becomes more negative as the particles get closer together (as r decreases). Therefore, opposite charges (like opposite poles on a magnet) attract each other. ■ The magnitude of the interaction between charged particles increases as the charges of the particles increase. Consequently, an electron with a charge of 1-is more strongly attracted to a nucleus with a charge of 2+ than it is to a nucleus with a charge of 1+.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
1390...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the ionization energy of the He+ ion in kJ/mol (this would be the second ionization energy of He). See Problem 8.107. The Bohr formula for the energy levels of an ion consisting of a...
-
A technique called photoelectron spectroscopy is used to measure the ionization energy of atoms. A sample is irradiated with UV light, and electrons are ejected from the valence shell. The kinetic...
-
In steel processing industry, structural steel products are fabricated by heating, rolling and then cooling process. A steel rod of cylindrical shape is at the uniform temperature of 900C at the end...
-
1. What responsibility does an organization have to ensure that its suppliers and business partners behave ethically? To whom is this responsibility owed? 2. How can an organization monitor the...
-
On December 31, 2009, Milo Company had 1,300,000 shares of $5 par common stock issued and outstanding. The stockholders equity accounts at December 31, 2009, had the balances listed here. Common...
-
The composite body shown is formed by removing a semi ellipsoid of revolution of semi major axis h and semi minor axis 2 a from a hemisphere of radius a. Determine (a) The y coordinate of the...
-
Continuing to focus on evidence associated with the act, concealment, and conversion, use the evidentiary material to continue the examination. In addition, the examiner also starts to think of terms...
-
Following are several decisions that the auditor must make in an audit of a nonpublic company. Letters indicate alternative conclusions that could be made. Required a. Identify the sequence in which...
-
Prove that f(xx, yy) = (i=1 (yy) (1/2) x) (i-1 (yy)(1/2)x}) where 1 and 2 refer to periods 1 and 2 respectively; satisfies the condition that: fk(ykx,ykk) = for all k=1,...K then f(yx,y)=\
-
Maria sighed as she considered her new assignment. It had seemed like a great idea when Iris offered her the role, but now she wondered if she could get her arms around the complex process of getting...
-
Write the electron configurations of the six cations that form from sulfur by the loss of one to six electrons. For those cations that have unpaired electrons, write orbital diagrams.
-
The elements with atomic numbers 35 and 53 have similar chemical properties. Based on their electronic configurations, predict the atomic number of a heavier element that also should share these...
-
Abramowitz obtained a one-year mortgage loan from Barnett Bank for $400,000 at 9 percent interest with a 1 percent "point" or service fee. The maximum lawful rate of interest on such a loan is 10...
-
Consider a particle whose position vector is given by thevec(r)(t) = Acos(wt)hat(y). following expression: ~r(t) = Acos(wt) (y cap) (y cap is the vector) Q2a: Describe in one sentence the motion of...
-
what ways does the Multilevel Feedback Queue (MLFQ) scheduling algorithm address the complexities of modern computing environments by incorporating multiple priority levels and feedback mechanisms?
-
Watch the film Mega Mall shown in class (retail development) (link provided on wk7, Canvas) and answer the following questions: Why was thePalisades Center, at Clark's town a controversial project?...
-
Exercise 1.8. How many different binary numbers can you write with: 4 bits 5 bits n bits I
-
How does the Rate Monotonic Scheduling (RMS) algorithm facilitate the predictable scheduling of periodic tasks in embedded systems by assigning priorities based on task periods?
-
Model 99 Hotels is considering the construction of a new hotel for $80 million. The expected life of the hotel is 20 years with no residual value. The hotel is expected to earn revenues of $15...
-
Which of the followingcarbocations is the least stable? CH3CH2 . CH3CHCH3 CH3 I . CH3C0 T CH3 IV. V. CH3 CH3CCH2 CH3
-
Show that the energy density radiated by a blackbody depends on the temperature as T 4 . The definite integral Using your result, calculate the energy density radiated by a blackbody at 1100. K and...
-
What speed does a F 2 molecule have if it has the same momentum as a photon of wavelength 225 nm?
-
A newly developed substance that emits 250. W of photons with a wavelength of 325 nm is mounted in a small rocket initially at rest in outer space such that all of the radiation is released in the...
-
The population in January 2 0 0 3 was estimated to be about 6 . 3 0 billion people. Assume that the population will continue to grow exponentially at the rate of about 1 . 3 % per year. Then the...
-
Consider the following logarithmic equation. log3(x) log.x 8- log(x) log(x) = 1. (i) Find the value(s) of a satisfying in the equation. (ii) Determine for what values of the logarithmic expression on...
-
The amount of bacteria in a culture was continuously increasing at a rate of 15%. The original bacteria count was 1000, and time, t, is measured in hours. 13. Write a model for the bacteria count in...

Study smarter with the SolutionInn App


