Use formal charge to choose the best Lewis structure for CH 3 SOCH 3 . H :O:
Question:
Use formal charge to choose the best Lewis structure for CH3SOCH3.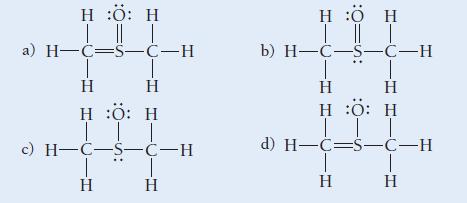
Transcribed Image Text:
H :O: H | || | a) H=C=5-C-H T Η Η H :O: H III © H-C-5-C-H Η Η H :O H | || | b) H-C-5-C-H Η Η H :O: H ||| d) H-C=s-C-H Η T Η
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
H O...View the full answer

Answered By

Mishark muli
Having any assignments and any other research related work? worry less for I am ready to help you with any task. I am quality oriented and dedicated always to produce good and presentable work for the client once he/she entrusts me with their work. i guarantee also non plagiarized work and well researched work to give you straight As in all your units.Feel free to consult me for any help and you will never regret
4.70+
11+ Reviews
37+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The algorithm below determines q, r EN such that y = q2 +r and r < 2, where y, z N. DIVIDE(y, z) 1 ry 2 q 0 3 4 5 6 7 8 9 10 11 12 while wy do w- 2w while w > z do q2q [w/2] W- if w
-
Briefly describe some common information system controls that need to be implemented by business managers, not IS professionals.
-
You have been assigned to a project to determine if a new investment should be made. your company uses a capital structure of 30% debt and 70% equity. the debt currently pays 8.5% interest; the...
-
Table illustrates the quantities, marginal costs, average variable costs, and average costs of a competitive firm. Refer to table 4. How much is the unit profit at price =$30? [the problem is based...
-
Describe some generic types of record keys in typical accounting databases. Are such keys simple or complicated?
-
The boron isotope 9 B is unstable and disintegrates into a proton and two particles. The total energy released as kinetic energy of the decay products is 4.4 10 14 J. In one such event, with the 9...
-
When the plaintiff was 16, he was employed by Kmart as a cashier. At the end of his training, he was required to read Kmarts policy agreement, which included an agreement to submit all employment...
-
The following selected transactions were completed by Green Lawn Supplies Co., which sells irrigation supplies primarily to wholesalers and occasionally to retail customers: July 1. Sold merchandise...
-
1)Consider the crowding out model. Assume there are men and women in the labor force and three occupations: preschool, elementary and secondary teachers. Suppose only women can work as elementary and...
-
Write a Lewis structure for the NO 3 ion. Include resonance structures.
-
The salinity of seawater can vary in the worlds oceans as shown in the map, which indicates salinity in units of percent by mass NaCl. Examine the image and answer the questions that follow. a. Which...
-
What main coverages are included in home insurance policies?
-
What are the similarities and differences between human marriages and interfirm alliances? How can the lessons behind the success and failure of human marriages enhance the odds of alliance success?
-
Suppose a golfer on the University of Houston golf team plays 70 rounds of golf and breaks par 32 times. Construct a 90 % confidence interval for the proportion of rounds in which this golfer will...
-
Your CPA firm is organizing a one-day-long CSR activity using company time such as cleaning up a dirty road or picking up trash on the beach. A colleague tells you: This is so stupid. I already have...
-
Founded in 1938, Samsung Group is South Koreas leading conglomerate. It has 320,000 employees in 510 units in 80 countries, with more than $300 billion in annual revenues in 2019. The flagship...
-
What are some of the darker sides associated with globalization? Why are negative attitudes toward globalization growing in some parts of the world? Since the swing of the pendulum is likely to move...
-
What is Good harts law? How is it relevant to (a) Monetary policy; (b) Using assignment grades to assess a students ability; (c) Paying workers according to the amount of output they produce; (d)...
-
The following cost information was provided to you for analysis: September 12,000 Units Produced Costs: TIC TAC TOE TING August 10,000 P80,000 70.000 60.000 50,000 How much is the fixed cost per...
-
Consider the plane electromagnetic wave in vacuum (in SI units) given by the expressions E x = 0, E y = 2 cos [2 10 14 (t - x/c) + /2], and E z = 0. (a) What are the frequency, wavelength, direction...
-
Write an expression for the vector E- and vector B-fields that constitute a plane harmonic wave traveling in the +z-direction. The wave is linearly polarized with its plane of vibration at 45 to the...
-
Considering Eq. (3.30), show that the expression is correct as it applies to a plane wave for which the direction of the electric field is constant. KxE= (3.30) Ey 3 .
-
Chapter: Life Policy Provisions, Riders and Options Question 6 of 15 Under which nonforfeiture option does the company pay the surrender value and have no further obligations to the policyowner?...
-
Describe what digital marketing is, using examples. Explain two types of digital strategies marketers use to gain and retain more customers. Support your discussion with a real-world example for each...
-
The goal is to focus/identify their instrument, sampling method, and treatment of data. Election poll survey results have since been an indication of possible election turnout. In the 2022...

Study smarter with the SolutionInn App


