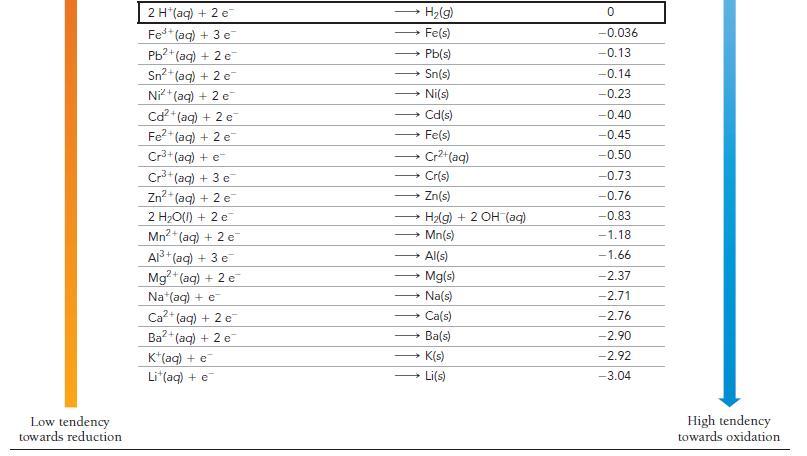
Use Table 20.1 to calculate E cell for the reaction. 2 C1O(g) + Pb(s) a) 1.77 V
Question:
Use Table 20.1 to calculate E°cell for the reaction.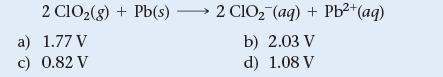
Transcribed Image Text:
2 C1O₂(g) + Pb(s) a) 1.77 V c) 0.82 V 2 ClO₂ (aq) + Pb²+ (aq) b) 2.03 V d) 1.08 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
d...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For the cell Pt(s) | VO2+(0.116 M), V3+(0.116 M), H+(1.57 M) || Sn2+(0.031 8 M), Sn4+(0.031 8 M) | Pt(s), E (not E ) = - 0.289 V. Write the net cell reaction and calculate its equilibrium constant....
-
Use data from Table 19.1 to predict whether, to any significant extent, (a) Mg(s) will displace Pb 2+ from aqueous solution; (b) Sn(s) will react with and dissolve in 1 M HCl; (c) SO 4 2- will...
-
Manganese may play an important role in chemical cycles in the oceans. Two reactions involving manganese (in acid solution) are the reduction of nitrate ions (to NO) with Mn 2+ ions and the oxidation...
-
The following is a comparative consolidated Statement of Financial Position for a particular firm X: Consolidated Balance Sheet (S in Millions) Current assets Cash and short-term investments Accounts...
-
Vegan, LLC, owns a chain of gourmet vegetarian take-out markets. Last month, Store Q generated the following information: sales, $890,000; direct materials, $220,000; direct labor, $97,000; variable...
-
Here are the budgets of Brandon Surgery Center for the most recent historical quarter (in thousands of dollars): The center assumes that all revenues and costs are variable and hence tied directly to...
-
Assuming the same data as given in problem 9, was the well in each case profitable? Discuss your answer. Problem 9:- Property cost (acquisition cost). Drilling cost (one well). Estimated completion...
-
Items 1 through 9 are selected questions of the type generally found in internal control questionnaires used by auditors to obtain an understanding of internal control in the sales and collection...
-
Two large conducting plates on insulating stands are placed a distance D = 50 cm apart, as shown at right. The inner surface of one has a charge density of +0; the other, - The charge density on the...
-
Which statement is true for voltaic cells? a) Electrons flow from the anode to the cathode. b) Electrons flow from the more negatively charged electrode to the more positively charged electrode. c)...
-
In electrochemistry, what kind of reaction can be driven by electricity?
-
What are the nine main dimensions used to describe cross-cultural differences?
-
Arbitrary Inc has a fixed asset threshold of $2,500 per item. They purchase 5 computers for $10,000. How should that purchase be treated?
-
We are evaluating a project that costs $832,000, has an eight-year life, and has no salvage value. Assume that depreciation is straight-line to zero over the life of the project. Sales are projected...
-
When Blair completed his general T1 tax return, he determined he had taxable income of $68,000. However, he then realized he also had to do an AMT calculation. Over the course of the year, Blair...
-
Chris has gotten divorced in 2020. He pays a lump sum payment of $100,000 for spousal support, and then doesn't have to pay any in the future. He must pay $1,000 per month in child support starting...
-
In what ways do mitochondrial dynamics, encompassing processes like fission, fusion, and mitophagy, regulate cellular bioenergetics, apoptosis, and aging, and how are these dynamics altered in...
-
A review of industry-wide data for the domestic wine manufacturing industry suggests the following industry supply function: Q = -7,000,000 + 400,000P - 2,000,000PL - 1,500,000PK + 1,000,000W Where Q...
-
Find the market equilibrium point for the following demand and supply functions. Demand: 2p = - q + 56 Supply: 3p - q = 34
-
A particle of mass 3.0 kg moves with a horizontal velocity of 20 m/s as shown in Figure P9.47.? (a) What is the angular momentum of the particle about an axis that runs through point P and is...
-
Two disks are located on an axle as shown in Figure P9.46. The lower disk is initially spinning at 50 rad/s, and the upper one is not spinning. The upper disk then falls onto the lower disk, and they...
-
Estimate the angular momentum of an airplane propeller that is spinning at 2000 rpm. Concerned with the magnitude of the angular momentum, not its sign.
-
Please internet search and look for information regarding make a report based on it and include you reference. sample ; Operations department is the foundational department of any origination. There...
-
After this week's lecture on leases and other legal issues in real estate, describe why a buyer of property would want an estoppel agreement signed by the existing landlord and tenant, how an...
-
Suppose that f(c) = 5, g(c)=-6, f'(c) = 9, and g'(c) = 5. Then what is - value of (f(x) x g(x)) at x = c?

Study smarter with the SolutionInn App


