Use the vant Hoff factors in Table 14.9 to calculate each colligative property: a. The melting point
Question:
Use the van’t Hoff factors in Table 14.9 to calculate each colligative property:
a. The melting point of a 0.100 m iron(III) chloride solution
b. The osmotic pressure of a 0.085 M potassium sulfate solution at 298 K
c. The boiling point of a 1.22% by mass magnesium chloride solution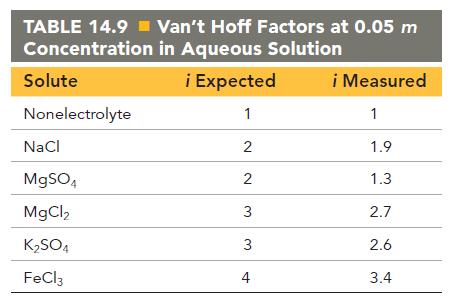
Transcribed Image Text:
TABLE 14.9 Van't Hoff Factors at 0.05 m Concentration in Aqueous Solution i Expected 1 Solute Nonelectrolyte NaCl MgSO4 MgCl, KSO4 FeCl3 2 2 3 3 4 i Measured 1 1.9 1.3 2.7 2.6 3.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a 063...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using the vant Hoff factors in Table 14.9, calculate the mass of solute required to make each aqueous solution: a. A sodium chloride solution containing 1.50 * 10 2 g of water that has a melting...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
How many orders of magnitude is 3 . 2 \ times 1 0 - 9 m smaller than 0 . 0 0 0 0 4 m ?
-
Jack Merritt is the controller for Universal Concrete Products (UCP), a manufacturing company with headquarters in Columbus, Ohio. UCP has seven concrete product plants located throughout the Midwest...
-
According to the pecking order theory, if additional external financing is required, what type of securities should a firm issue first? Last?
-
Saturn Corporation issued \(\$ 400,000\) of \(6 \%\) bonds payable on June 30. The bonds were dated April 30, and the semiannual interest dates are April 30 and October 31. 1. How much cash will...
-
The office currently has the following full-time equivalents (FTEs): 1.5 surgeons, 2 ASRs, 2RNs, and 1 CA. Suppose that in the following year, the office will have 5,400 plagiocephaly visits, 2,000...
-
1. Justin is saving for his retirement 25 years from now by setting up a savings plan. He has set up a savings plan wherein he will deposit $ 115.00 at the end of every six months for the next 14...
-
A 1.2 m aqueous solution of an ionic compound with the formula MX 2 has a boiling point of 101.4 C. Calculate the vant Hoff factor (i) for MX 2 at this concentration.
-
Determine the required concentration (in percent by mass) for an aqueous ethylene glycol (C 2 H 6 O 2 ) solution to have a boiling point of 104.0 C.
-
Derivation of the equation of continuity, in S19.1 the species equation of continuity is derived by making a mass balance on a small rectangular volume x y z fixed in space. (a) Repeat the derivation...
-
Consider a perfectly competitive firm with the following cost function: TC = 2(Q) + 1600 The firm sells its output for $168 per unit. A. B. C. D. Find the profit-maximizing level of output. Represent...
-
Requirement 1. For each depreciation method, prepare a depreciation schedule showing asset cost, depreciation expense, accumulated depreciation, and asset book value for each year of the asset's...
-
Reflect on your readings and the Biblical Perspective as you think through the following questions: Is it wrong for a business to seek to maximize profits? What does the Bible say about earning...
-
modify your code to run in the IDE's Node.js environment. The output of your program running in the Node.js environment should be similar to Figure 1 (i.e. individually list each element of the given...
-
1. A supernet has a first address of 205.17.64.0 and a supernet mask of 255.255.224.0. a. How many blocks are in this supernet and what is the range of addresses of each block? (15 points) b. A...
-
Describe the rationale behind using job order costing and process costing. Provide examples of three types of companies that would use each.
-
Revol Industries manufactures plastic bottles for the food industry. On average, Revol pays $76 per ton for its plastics. Revol's waste-disposal company has increased its waste-disposal charge to $57...
-
Light having a vacuum wavelength of 600 nm, traveling in a glass (n g = 1.50) block, is incident at 45 on a glassair interface. It is then totally internally reflected. Determine the distance into...
-
Derive an expression for the speed of the evanescent wave in the case of internal reflection. Write it in terms of c, n i , and i .
-
A large block of diamond is covered, on top, by a layer of water. A narrow beam of light travels upward in the solid and strikes the solidliquid interface. Determine the minimum incident angle that...
-
Create an infographic for Canadian Tire, based on industry benchmarks and its financial performance. Create an Infographic (visual representation of data) of the most important indicators of the...
-
Form an argument that mergers and acquisitions do not increase value for shareholders of the acquiring firm. You are talking with your work colleagues about the benefits of mergers and acquisitions....
-
Your tax client, Yankee LLC (the Company) was organized as a limited liability company under the laws of Virginia, effective January 1, 2020. The Company immediately elected to be taxed as an S...

Study smarter with the SolutionInn App


