What is the molarity of a 6.56% by mass glucose (C 6 H 12 O 6 )
Question:
What is the molarity of a 6.56% by mass glucose (C6H12O6) solution? (The density of the solution is 1.03 g/mL.)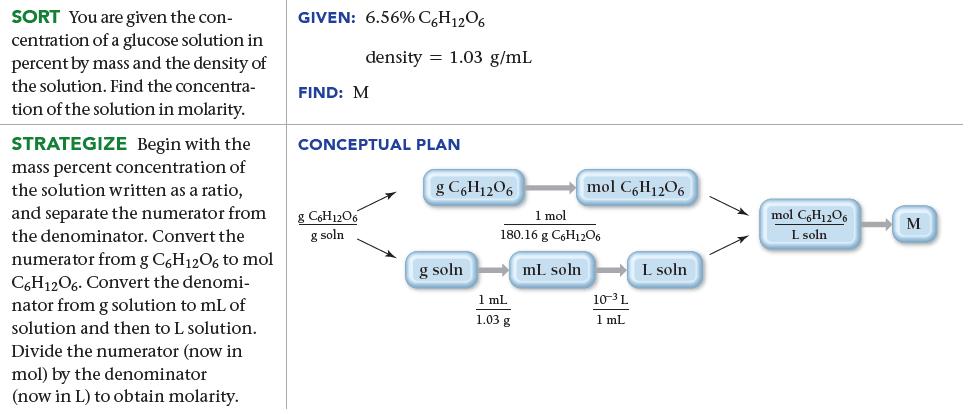
Transcribed Image Text:
SORT You are given the con- centration of a glucose solution in percent by mass and the density of the solution. Find the concentra- tion of the solution in molarity. STRATEGIZE Begin with the mass percent concentration of the solution written as a ratio, and separate the numerator from the denominator. Convert the numerator from g C6H12O6 to mol C6H12O6. Convert the denomi- nator from g solution to mL of solution and then to L solution. Divide the numerator (now in mol) by the denominator (now in L) to obtain molarity. GIVEN: 6.56% C6H12O6 density 1.03 g/mL FIND: M CONCEPTUAL PLAN 8 C6H12O6 g soln g C6H12O6 g soln mol C6H1206 1 mol 180.16 g C6H1206 ml. soln 1 mL 1.03 g 10-³L 1 mL L soln mol C6H1206 L soln M
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
656 g C6H1206 x 100 g soln X 1 m...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
What is the molarity of a solution made when 66.2 g of C6H12O6 are dissolved to make 235 mL of solution?
-
What is the molarity of a solution made by dissolving 332 g of C6H12O6 in 4.66 L of solution?
-
Show the parse trees for the two parses that the grammar assigns for sentence S1. S1: the train station bus rumbles [3 marks] (b) Give an algorithm for a bottom-up passive chart parser without...
-
What are the three viewpoints of product life cycle? How do they differ?
-
One of Jupiters moons, Io, has a mean orbital radius of 4.22 x 10 8 m and a period of 1.53 x 10 5 s. (a) Find the mean orbital radius of another of Jupiters moons, Callisto, whose period is 1.44 x 10...
-
Given the background reading, list three verbal deception and three non-verbal deception cues that you believe that Aldrich Ames might exhibit and describe why
-
The Sanding Department of Ortiz Furniture Company has the following production and manufacturing cost data for March 2010, the first month of operation. Production: 12,000 units finished and...
-
Display Oracle database employee last_name and salary for employee_ids between 100and 102. Include a third column that divides each salary by 1.55 and rounds the result two decimal places.
-
A solution contains 22.4 g glucose (C 6 H 12 O 6 ) dissolved in 0.500 L of water. What is the molality of the solution? (Assume a density of 1.00 g/mL for water.) a) 0.238 m b) 44.8 m c) 0.249 m d)...
-
A solution is prepared by dissolving 17.2 g of ethylene glycol (C 2 H 6 O 2 ) in 0.500 kg of water. The final volume of the solution is 515 mL. Calculate the concentration of the solution in each...
-
Prove that if A is a regular 2 Ã 2 matrix, then its L U factorization is unique. In other words, if where L, are special lower triangular and are upper triangular, then and A=LU = LU where L,L
-
At the beginning of the year, NRD Company purchased the rights to a natural resource for $10,000,000. The estimated recoverable units from the natural resource amount to 3,500,000 units. During the...
-
The current trend in the use of staff in organizations is to ____________. (a) give staff personnel more authority over operations (b) reduce the number of staff personnel (c) remove all staff from...
-
Beth, who is single, redeems her Series EE bonds. She receives $12,000, consisting of $8,000 principal and $4,000 interest. Beth's qualified educational expenses total $16,500. Further, Beth's...
-
On January 4, 2019, Ralph Stuart, an employee of Hard Manufacturing Inc., enrolled for the spring semester at State University where he is a candidate for an undergraduate degree in accounting. His...
-
Mindy is a candidate for a bachelors degree at a local state university. She received a grant that covered the following expenses: Mindys tuition this year was $8,000. She spent the entire $7,000...
-
Harmons has several departments that occupy all floors of a two- story building that includes a basement floor. Harmon rented this building under a long-term lease negotiated when rental rates were...
-
Use the information given about the angles and to find the exact value of: (a) sin( + ) (b) cos( + ) (c) sin( - ) (d) tan ( + ) (e) sin(2) (f) cos (2) (g) sin /2 (h) cos/2 cos = 4/5, 0 < < /2; cos =...
-
Oil with a specific gravity of 0.90 is flowing downward through the venturi meter shown in Fig. 6.33. If the velocity of flow in the 2-in-diameter section is 10.0 ft/s, calculate the deflection h of...
-
Oil with a specific gravity of 0.90 is flowing downward through the venturi meter shown in Fig. 6.33. If the manometer deflection h is 28 in, calculate the volume flow rate of oil. 4-in inside...
-
The venturi meter shown in Fig. 6.32 carries oil (sg = 0.90). The specific gravity of the gage fluid in the manometer is 1.40. Calculate the volume flow rate of oil. 75-mm inside diameter Flow 0.25 m...
-
Create 8 to 10 page marketing plan for Accurate Handyman Solutions, small new company Write in business report format using full paragraphs unless otherwise stated. It is crucial to follow various...
-
Solve the equation for b. 2b2+21b+54=0
-
Solve: log2 (x-2)-log2 (6x-5)= 0.

Study smarter with the SolutionInn App


