When 1.010 g of sucrose (C 12 H 22 O 11 ) undergoes combustion in a bomb
Question:
When 1.010 g of sucrose (C12H22O11) undergoes combustion in a bomb calorimeter, the temperature rises from 24.92 °C to 28.33 °C. Find ΔErxn for the combustion of sucrose in kJ/mol sucrose. The heat capacity of the bomb calorimeter, determined in a separate experiment, is 4.90 kJ/°C.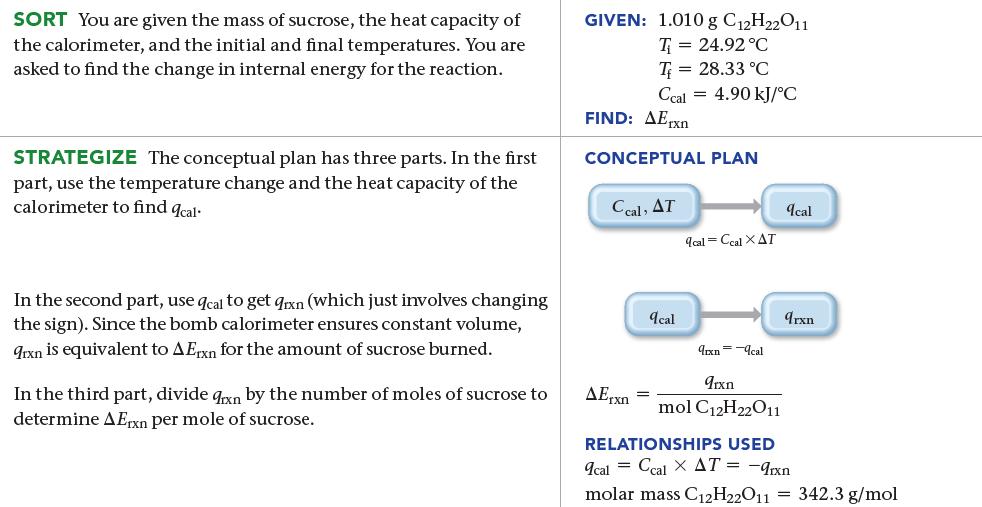
Transcribed Image Text:
SORT You are given the mass of sucrose, the heat capacity of the calorimeter, and the initial and final temperatures. You are asked to find the change in internal energy for the reaction. STRATEGIZE The conceptual plan has three parts. In the first part, use the temperature change and the heat capacity of the calorimeter to find 9cal. In the second part, use qcal to get aixn (which just involves changing the sign). Since the bomb calorimeter ensures constant volume, 9rxn is equivalent to A Exn for the amount of sucrose burned. In the third part, divide qxn by the number of moles of sucrose to determine AErxn per mole of sucrose. GIVEN: 1.010 g C12H22011 T= 24.92 °C T= 28.33 °C Ccal FIND: AErxn CONCEPTUAL PLAN Ccal, AT AErxn = 4.90 kJ/°C qcal qcal Ccal XAT 9rxn=cal 9rxn mol C12H22011 qcal 9rxn RELATIONSHIPS USED 9cal = ccal X AT = -9rxn molar mass C₁2H22011 = 342.3 g/mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
T T T 2833 C 2492 C 341 C qcal Ccal X AT ...View the full answer

Answered By

Arshad Ahmad
Well, I am really new to tutoring but I truly believe a good student can be a better teacher. I have always been a topper at school. I passed my Chartered Accountancy at a very young age of 23, a rare feat for most of the students. I am really dedicated to whatever work I do and I am very strict regarding deadlines. i am always committed and dedicated to whatever work allotted to me and I make sure it is completed well within deadline and also I try to give my best in whatever I do. Hope we will have a good time studying together.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Define pay iniquity
-
When 1.10 g of ethanol (C 2 H 6 O) undergoes combustion in a bomb calorimeter, the temperature rises from 22.32C to 29.48C. Find E rxn for the combustion of ethanol in kJ/mol. The heat capacity of...
-
When 0.514 g of biphenyl (C 12 H 10 ) undergoes combustion in a bomb calorimeter, the temperature rises from 25.8 C to 29.4 C. Find E rxn for the combustion of biphenyl in kJ/mol biphenyl. The heat...
-
Consider a social network where people are represented as vertices and friendships as edges. If there are 1 0 people in the network and each person is friends with 3 others, calculate the total...
-
Morey Company has just completed its first year of operations. The companys absorption costing income statement for the year appears below: The companys selling and administrative expenses consist of...
-
Value chain and classification of costs computer company, Company Computer incurs the following costs: a. Electricity costs for the plant assembling the Presario computer line of products b....
-
A Pepsi promotion encouraged consumers to collect Pepsi points and redeem them for merchandise. If they did not have quite enough points for the prize they wanted, they could buy additional points...
-
Long-Term Contract with Interim Loss On March 1, 2010, Pechstein Construction Company contracted to construct a factory building for Fabrik Manufacturing Inc. for a total contract price of...
-
Create an interface, Stock derivatives, with two attributes viz., delta=0.5 and vega=0.2 which are used to determine option premium of a stock. Also add an abstract method Find Premium. Write a class...
-
From a molecular viewpoint, where does the energy absorbed in an endothermic chemical reaction go? Why does the reaction mixture undergo a decrease in temperature even though energy is absorbed?
-
A 32.5 g cube of aluminum initially at 45.8 C is submerged into 105.3 g of water at 15.4 C. What is the final temperature of both substances at thermal equilibrium? (Assume that the aluminum and the...
-
Which of the following is not one of the presumed components of happiness? a. Using our talents to help improve the lives of others b. Learning new skills c. Regular pleasurable experiences d....
-
Should the component cost estimates reflect historical or marginal costs?
-
Mr. Girdhari Lal does not keep full double entry records. His balance as on January 01, 2006 is as. He withdrew Rs. 500 per month out of which to spent Rs. 1,500 for business purpose. Prepare the...
-
What are some roles that investment bankers play in mergers?
-
What is the general formula for the corporate cost of capital?
-
What are the differences between the waves of the 1980s and the 1990s?
-
In 2010, Tarlo Company agrees to construct a highway for Brice County over a three-year period (2010 through 2012). The contract price is $1,200,000 and the construction costs (both actual and...
-
A business had revenues of $280,000 and operating expenses of $315,000. Did the business (a) Incur a net loss (b) Realize net income?
-
Consider the reaction: 2 COF 2 (g) CO 2 (g) + CF 4 (g) Kc = 2.00 In an equilibrium mixture, the concentration of COF 2 is 0.35 M and the concentration of CO 2 is 0.144 M. What is the equilibrium...
-
Consider the reaction: N 2 O 4 (g) 2 NO 2 (g) Kc = 0.36 A reaction mixture initially contains [N 2 O 4 ] = 0.100 M. Find the equilibrium concentrations of N 2 O 4 and NO 2 .
-
Consider the reaction: 2 H 2 S (g) 2 H 2 (g) + S 2 (g) Kc = 1.67 10 -7 A reaction mixture initially contains [H 2 S] = 0.010 M. Find the equilibrium concentrations of H 2 and S 2 .
-
Empire, Inc. currently has an inventory of 100 74-z Speeder Bikes left from last year. Also last year, they sold 90 bikes for $50 per speeder. What is the value of output that would be included in...
-
You need to adjust a comparable property price by 15% to match the home you're trying to list. If the sales price for the home was $185,000, what's the adjusted price?
-
What would you conclude about the trends in admissions for St. Mary's Hospital for calendar year 2018? Sample Frequency Distribution of Admissions to St. Mary's Hospital Per Month for Calendar Year...

Study smarter with the SolutionInn App


