When fuels are burned in air, such as in an automobile engine, some of the nitrogen in
Question:
When fuels are burned in air, such as in an automobile engine, some of the nitrogen in the air oxidizes to form nitrogen oxide gases such as NO and NO2 (known collectively as NOx). The U.S. Environmental Protection Agency (EPA) sets standards for air quality of several pollutants including NO2. According to the EPA, NO2 levels in U.S. cities are not to exceed a yearly average of 53 ppb or a 1-hour average of 100 ppb. Another pollutant associated with automobile exhaust is ozone (O3). The EPA standard for ozone is an 8-hour average of 70 ppb. Breathing air with elevated levels of NO2 or O3 can cause asthma and other respiratory problems. The graph shown here shows the average concentration of nitrogen dioxide (NO2) and ozone (O3) gases in units of parts per billion by volume (ppbv) over seven days in a city.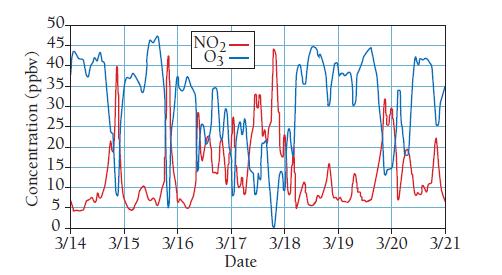
a. What type of relationship exists between nitrogen dioxide and ozone between March 14 and March 16?
b. Calculate the number of moles of NO2 in 1.00 m3 produced on March 14. Assume an average temperature of 25.0 °C and a pressure of 1 atm. Note that the number of moles of NO2 produced is the difference between the existing amount at the start of the day and the peak amount.
c. Calculate the number of moles of O3 in 1.00 m3 consumed on March 14. Assume an average temperature of 25.0 °C and a pressure of 1 atm. Note that the number of moles of O3 consumed is the difference between the existing amount at the start of the day and the minimum amount.
d. What is the mole-to-mole ratio of O3 consumed to NO2 produced?
e. The following chemical equations model the interactions of nitrogen dioxide gas and ozone gas. Can this set of equations account for the trends observed in the graph?
Explain your answer.![]()
f. Do the concentrations of NO2 or O3 exceed the standards set by the EPA?
Step by Step Answer:






