When hydrazine is dissolved in water, it acts like a base. a. Calculate the Kb for the
Question:
When hydrazine is dissolved in water, it acts like a base.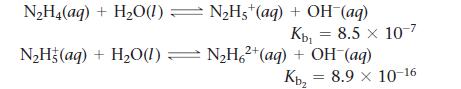
a. Calculate the Kb for the overall reaction of hydrazine forming N2H62+.
b. Calculate Ka1 for N2H5+.
c. Calculate the concentration of hydrazine and both cations in a solution buffered at a pH of 8.5 for a solution that was made by dissolving 0.012 mol of hydrazine in 1 L of water.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





