When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. a. Is the dissolution of
Question:
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter.
a. Is the dissolution of lithium iodide endothermic or exothermic?
b. What can you conclude about the relative magnitudes of the lattice energy of lithium iodide and its heat of hydration?
c. Sketch a qualitative energy diagram similar to Figure 14.7 for the dissolution of LiI.
d. Why does the solution form? What drives the process?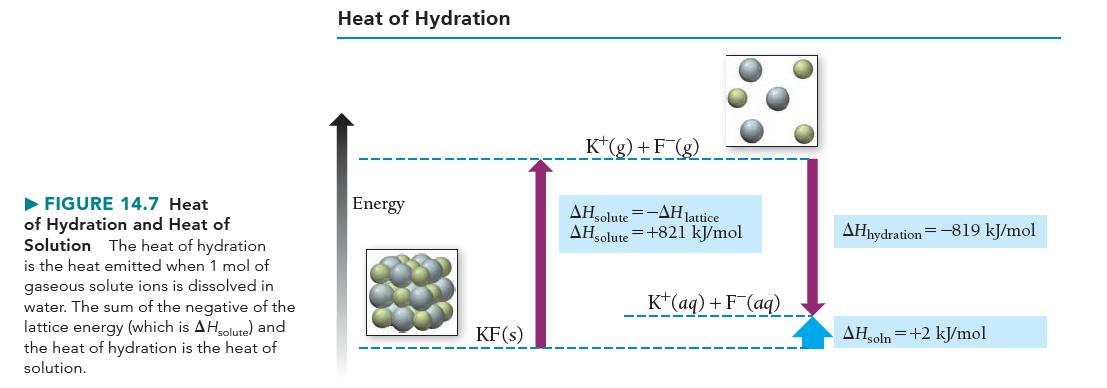
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





