Which of these two reactions would you expect to have the smaller orientation factor? Explain. a. O(g)
Question:
Which of these two reactions would you expect to have the smaller orientation factor? Explain.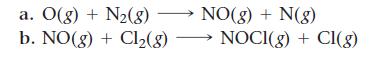
Transcribed Image Text:
a. O(g) + N₂(8) NO(g) + N(g) b. NO(g) + Cl₂(g) → NOCI(g) + Cl(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
The orientation factor or steric factor is a term used in collision theory to account for the probab...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider two hypothetical elements, W and Z. Element W has an electron affinity of 300 kJ/mol, and element Z has an electron affinity of 75 kJ/mol. a. If you have a W ion and a Z ion, from which ion...
-
Which of the following reactions would you expect to have the larger rate at room temperature? Why? (Hint: Think of which would have the lower activation energy.) 2Ce4+(aq) + Hg22+(aq) 2Ce3+(aq) +...
-
Consider these two gas-phase reactions: a. AA(g) + BB(g) 2 AB( g) b. AB(g) + CD(g) AC(g) + BD(g) If the reactions have identical activation barriers and are carried out under the same conditions,...
-
Write a program that takes an integer command-line argument n and prints all the positive powers of 2 less than or equal to n. Make sure that your program works properly for all values of n.
-
Langdon Company produced 9,000 units during the past year, but only 8,200 of the units were sold. The following additional information is also available. Direct materials used .......... $79,000...
-
The following is a summary of all relevant transactions of Vicario Corporation since it was organized in 2012. In 2012, 15,000 shares were authorized and 7,000 shares of common stock ($50 par value)...
-
Briefly explain how the following programs would affect the elasticity of demand for labor in the steel industry: a. An increased tariff on steel imports. b. A law making it illegal to lay off...
-
In recent years, Harper Transportation purchased three used buses. Because of frequent employee turnover in the accounting department, a different accountant selected the depreciation method for each...
-
3. (25 points) Consider two firms out of a competitive industry. They have the following technologies: C(y) = y + 2y; C2(y) = 1.5y + 3y. Show these firms' individual supply functions on a...
-
The proposed mechanism for the formation of hydrogen bromide can be written in a simplified form as: What rate law corresponds to this mechanism? Br(g) k 2Br(g) Br(g) + H(g) H(g) + Br(g) k3 HBr(g) +...
-
If a temperature increase from 20.0 C to 35.0 C triples the rate constant for a reaction, what is the value of the activation barrier for the reaction?
-
Experiment shows that the first ionization energy of lithium is less than the first ionization energy of beryllium, but the second ionization energy of beryllium is less than the second ionization...
-
What are the characteristics of the transnational model?
-
What is the difference between global account management and global solution selling?
-
What are the six domains that need to be negotiated in relation to the interface and governance of an alliance?
-
Jack Cumberland was the Managing Director of IndoMedia, a leading advertising agency in [the fictional Southeast-Asian country] Indoland. IndoMedia was formed as a joint venture between EuroMedia...
-
What are the typical problems associated with global account management?
-
On January 1, 2013, Ward Corp. issued $160,000 of 10-year, 7 percent bonds at their face amount. Interest is payable on December 31 of each year with the first payment due December31, 2013. Required...
-
(a) Find the equation of the tangent line to f(x) = x 3 at the point where x = 2. (b) Graph the tangent line and the function on the same axes. If the tangent line is used to estimate values of the...
-
Design a problem to help other students better understand obtaining the Fourier series from a periodic function. A periodic function is defined over its period as Find the Fourier series of h(t). (10...
-
Using Fig. 17.51 , design a problem to help other students better understand how to determine the exponential Fourier series from a periodic wave shape. Obtain the exponential Fourier series of the...
-
Find the trigonometric Fourier series for 7.5 0
-
What is the value of Z if only 9.68% of all possible Z values are greater? Z=?
-
1. To determine the proportion of voters who favor a certain candidate for governor, the campaign staff phones 2500 residents of the state chosen from the state property tax rolls. The 2500 residents...
-
Divide the rational expression. Simplify. Q16 a-1 a+6 +1

Study smarter with the SolutionInn App


